FOXM1 regulates glycolysis in nasopharyngeal carcinoma cells through PDK1
- PMID: 35656815
- PMCID: PMC9258706
- DOI: 10.1111/jcmm.17413
FOXM1 regulates glycolysis in nasopharyngeal carcinoma cells through PDK1
Abstract
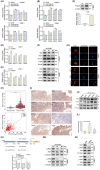
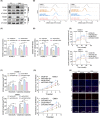
The transcription factor forkhead box M1 (FOXM1) is a well-known proto-oncogene that plays a significant role in the pathogenesis of various human cancers. However, the regulatory role and underlying mechanisms of FOXM1 in nasopharyngeal carcinoma (NPC) metabolism remain unclear. We demonstrated that FOXM1 could positively regulate glycolysis in NPC cells. Functional studies have shown that pyruvate dehydrogenase kinase 1 (PDK1) is involved in FOXM1-regulated lactate production, ATP generation and glycolysis. FOXM1 binds directly to the PDK1 promoter region and increases the expression of PDK1 at the transcriptional level, leading to the phosphorylation of pyruvate dehydrogenase (PDH) at serine 293, inhibiting its activity. Knocking down FOXM1 using specific short hairpin RNAs (shRNAs) can significantly decrease glycolysis and the expression of PDK1 in NPC cells. Furthermore, microenvironmental factors can increase the expression of FOXM1 by regulating hypoxia-inducible factor 1α (HIF-1α) expression. Clinical data and in vivo studies confirmed the positive roles of FOXM1/PDK1 in NPC proliferation and progression. In conclusion, our findings revealed that FOXM1 regulates glycolysis and proliferation of NPC through PDK1-mediated PDH phosphorylation. Therefore, targeting the FOXM1-PDK1 axis may be a potential therapeutic strategy for NPC.
Keywords: FOXM1; PDK1; glycolysis; nasopharyngeal carcinoma; proliferation.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64‐80. - PubMed
-
- Lai SZ, Li WF, Chen L, et al. How does intensity‐modulated radiotherapy versus conventional two‐dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661‐668. - PubMed
-
- Elia I, Doglioni G, Fendt SM. Metabolic hallmarks of metastasis formation. Trends Cell Biol. 2018;28(8):673‐684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

