CTRP12 alleviates cardiomyocyte ischemia‑reperfusion injury via regulation of KLF15
- PMID: 35656890
- PMCID: PMC9185681
- DOI: 10.3892/mmr.2022.12763
CTRP12 alleviates cardiomyocyte ischemia‑reperfusion injury via regulation of KLF15
Abstract
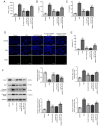
Myocardial ischemia‑reperfusion (I/R) serves a crucial role in myocardial infarction. C1q/TNF‑related protein 12 (CTRP12) is a secretory protein involved in metabolism. It has been reported that CTRP12 participates in the regulation of numerous cardiovascular diseases. However, its role in myocardial I/R injury remains unclear. In the present study, the left anterior descending coronary artery in mice was ligated to establish a mouse I/R model. A myocardial hypoxia‑reoxygenation (H/R) cell model was also established. Cardiomyocyte injury was evaluated using hematoxylin and eosin staining, Cell Counting Kit‑8 and a lactate dehydrogenase (LDH) kit. The expression levels of CTRP12 and Krueppel‑like factor 15 (KLF15) in murine myocardial tissues and H9c2 cells were determined using reverse transcription‑quantitative PCR and western blotting, as KLF15 was previously reported to protect against I/R‑induced cardiomyocyte damage. Furthermore, inflammatory factors TNF‑α, IL‑1β and IL‑6 were analyzed using ELISA while apoptosis was assessed using TUNEL assays and western blotting. Moreover, the activity of the CTRP12 promoter was determined using a dual‑luciferase reporter assay. The results demonstrated that I/R surgery markedly exacerbated myocardial tissue damage, whereas H/R treatment significantly reduced cell viability and significantly increased LDH activity as well as the release of inflammatory factors and apoptosis. I/R and H/R induction significantly reduced the expression levels of CTRP12 and KLF15. CTRP12 overexpression significantly alleviated H/R‑induced cell injury and significantly inhibited inflammation and apoptosis. Further analysis demonstrated that KLF15 could significantly promote the activity of the CTRP12 promoter. However, following CTRP12 knockdown, KLF15 overexpression exacerbated cell injury, inflammation and apoptosis. In conclusion, the present study demonstrated that CTRP12 may mitigate inflammation and apoptosis in H/R‑induced cardiomyocytes, possibly via the regulation of KLF15, which provided a theoretical basis for the potential treatment of I/R‑induced myocardial infarction.
Keywords: C1q/tumor necrosis factor‑related protein 12; Krueppel‑like factor 15; apoptosis; inflammation; myocardial ischemia‑reperfusion injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction: A Narrative Review.Int J Mol Sci. 2025 May 18;26(10):4831. doi: 10.3390/ijms26104831. Int J Mol Sci. 2025. PMID: 40429972 Free PMC article. Review.
-
Up-regulation of CTRP12 ameliorates hypoxia/re-oxygenation-induced cardiomyocyte injury by inhibiting apoptosis, oxidative stress, and inflammation via the enhancement of Nrf2 signaling.Hum Exp Toxicol. 2021 Dec;40(12):2087-2098. doi: 10.1177/09603271211021880. Epub 2021 Jun 4. Hum Exp Toxicol. 2021. PMID: 34085554
-
Lycium barbarum polysaccharide protects rats and cardiomyocytes against ischemia/reperfusion injury via Nrf2 activation through autophagy inhibition.Mol Med Rep. 2021 Nov;24(5):778. doi: 10.3892/mmr.2021.12418. Epub 2021 Sep 9. Mol Med Rep. 2021. PMID: 34498711 Free PMC article.
-
Remimazolam alleviates myocardial ischemia/reperfusion injury and inflammation via inhibition of the NLRP3/IL‑1β pathway in mice.Int J Mol Med. 2025 Apr;55(4):57. doi: 10.3892/ijmm.2025.5498. Epub 2025 Jan 31. Int J Mol Med. 2025. PMID: 39886966 Free PMC article.
-
MicroRNA‑221‑3p contributes to cardiomyocyte injury in H2O2‑treated H9c2 cells and a rat model of myocardial ischemia‑reperfusion by targeting p57.Int J Mol Med. 2018 Jul;42(1):589-596. doi: 10.3892/ijmm.2018.3628. Epub 2018 Apr 17. Int J Mol Med. 2018. PMID: 29693157
Cited by
-
The G protein-coupled receptor ligand apelin-13 ameliorates skeletal muscle atrophy induced by chronic kidney disease.J Cachexia Sarcopenia Muscle. 2023 Feb;14(1):553-564. doi: 10.1002/jcsm.13159. Epub 2022 Dec 23. J Cachexia Sarcopenia Muscle. 2023. PMID: 36562292 Free PMC article.
-
Complement 1q/Tumor Necrosis Factor-Related Proteins (CTRPs): Structure, Receptors and Signaling.Biomedicines. 2023 Feb 14;11(2):559. doi: 10.3390/biomedicines11020559. Biomedicines. 2023. PMID: 36831095 Free PMC article. Review.
-
PCSK9 Inhibitor Inclisiran Attenuates Cardiotoxicity Induced by Sequential Anthracycline and Trastuzumab Exposure via NLRP3 and MyD88 Pathway Inhibition.Int J Mol Sci. 2025 Jul 10;26(14):6617. doi: 10.3390/ijms26146617. Int J Mol Sci. 2025. PMID: 40724868 Free PMC article.
-
Postoperative evaluation of interleukin-8 and C1q/TNF-related protein-12 in patients undergoing coronary artery bypass graft surgery.Rev Assoc Med Bras (1992). 2025 Jan 10;71(1):e20240877. doi: 10.1590/1806-9282.20240877. eCollection 2025. Rev Assoc Med Bras (1992). 2025. PMID: 39813442 Free PMC article.
-
Effect of KLF15-Mediated Circadian Rhythm on Myocardial Infarction: A Narrative Review.Int J Mol Sci. 2025 May 18;26(10):4831. doi: 10.3390/ijms26104831. Int J Mol Sci. 2025. PMID: 40429972 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

