Small Molecule NIR-II Dyes for Switchable Photoluminescence via Host -Guest Complexation and Supramolecular Assembly with Carbon Dots
- PMID: 35657032
- PMCID: PMC9353451
- DOI: 10.1002/advs.202202414
Small Molecule NIR-II Dyes for Switchable Photoluminescence via Host -Guest Complexation and Supramolecular Assembly with Carbon Dots
Abstract
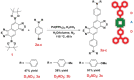
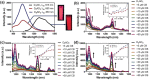
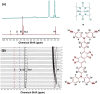
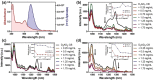
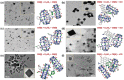
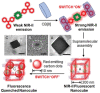
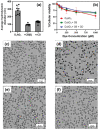
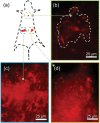
Small molecular NIR-II dyes are highly desirable for various biomedical applications. However, NIR-II probes are still limited due to the complex synthetic processes and inadequate availability of fluorescent core. Herein, the design and synthesis of three small molecular NIR-II dyes are reported. These dyes can be excited at 850-915 nm and emitted at 1280-1290 nm with a large stokes shift (≈375 nm). Experimental and computational results indicate a 2:1 preferable host-guest assembly between the cucurbit[8]uril (CB) and dye molecules. Interestingly, the dyes when self-assembled in presence of CB leads to the formation of nanocubes (≈200 nm) and exhibits marked enhancement in fluorescence emission intensity (Switch-On). However, the addition of red carbon dots (rCDots, ≈10 nm) quenches the fluorescence of these host-guest complexes (Switch-Off) providing flexibility in the user-defined tuning of photoluminescence. The turn-ON complex found to have comparable quantum yield to the commercially available near-infrared fluorophore, IR-26. The aqueous dispersibility, cellular and blood compatibility, and NIR-II bioimaging capability of the inclusion complexes is also explored. Thus, a switchable fluorescence behavior, driven by host-guest complexation and supramolecular self-assembly, is demonstrated here for three new NIR-II dyes.
Keywords: NIR-II emission; carbon dots; host-guest complexation; supramolecular assembly; switchable fluorescence.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Keereweer S., Van Driel P. B., Snoeks T. J., Kerrebijn J. D., de Jong R. J. B., Vahrmeijer A. L., Sterenborg H. J., Löwik C. W., Clin. Cancer Res. 2013, 19, 3745. - PubMed
-
- Pan D., Pramanik M., Wickline S. A., Wang L. v., Lanza G. M., Contrast Media Mol. Imaging 2011, 6, 378. - PubMed
-
- Mukherjee P., Misra S. K., Gryka M. C., Chang H.‐H., Tiwari S., Wilson W. L., Scott J. W., Bhargava R., Pan D., Small 2015, 11, 4691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
