Future directions for precision oncology in prostate cancer
- PMID: 35657153
- PMCID: PMC9942493
- DOI: 10.1002/pros.24354
Future directions for precision oncology in prostate cancer
Abstract
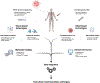
Clinical genomic testing is becoming routine in prostate cancer, as biomarker-driven therapies such as poly-ADP ribose polymerase (PARP) inhibitors and anti-PD1 immunotherapy are now approved for select men with castration-resistant prostate cancer harboring alterations in DNA repair genes. Challenges for precision medicine in prostate cancer include an overall low prevalence of actionable genomic alterations and a still limited understanding of the impact of tumor heterogeneity and co-occurring alterations on treatment response and outcomes across diverse patient populations. Expanded tissue-based technologies such as whole-genome sequencing, transcriptome analysis, epigenetic analysis, and single-cell RNA sequencing have not yet entered the clinical realm and could potentially improve upon our understanding of how molecular features of tumors, intratumoral heterogeneity, and the tumor microenvironment impact therapy response and resistance. Blood-based technologies including cell-free DNA, circulating tumor cells (CTCs), and extracellular vesicles (EVs) are less invasive molecular profiling resources that could also help capture intraindividual tumor heterogeneity and track dynamic changes that occur in the context of specific therapies. Furthermore, molecular imaging is an important biomarker tool within the framework of prostate cancer precision medicine with a capability to detect heterogeneity across metastases and potential therapeutic targets less invasively. Here, we review recent technological advances that may help promote the future implementation and value of precision oncology testing for patients with advanced prostate cancer.
Keywords: integrative analysis; molecular imaging; molecular profiling; precision oncology; prostate cancer.
© 2022 Wiley Periodicals LLC.
Figures
References
-
- Massard C, Michiels S, Ferté C, et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discovery. 2017;7(6):586–595. doi: 10.1158/2159-8290.cd-16-1396 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

