Evolution and Single-Droplet Analysis of Fuel-Driven Compartments by Droplet-Based Microfluidics
- PMID: 35657164
- PMCID: PMC9400878
- DOI: 10.1002/anie.202203928
Evolution and Single-Droplet Analysis of Fuel-Driven Compartments by Droplet-Based Microfluidics
Abstract
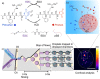
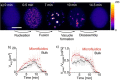
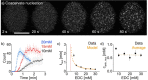
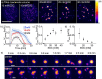
Active droplets are a great model for membraneless organelles. However, the analysis of these systems remains challenging and is often limited due to the short timescales of their kinetics. We used droplet-based microfluidics to encapsulate a fuel-driven cycle that drives phase separation into coacervate-based droplets to overcome this challenge. This approach enables the analysis of every coacervate-based droplet in the reaction container throughout its lifetime. We discovered that the fuel concentration dictates the formation of the coacervate-based droplets and their properties. We observed that coacervate-based droplets grow through fusion, decay simultaneously independent of their volume, and shrinkage rate scales with their initial volume. This method helps to further understand the regulation of membraneless organelles, and we believe the analysis of individual coacervate-based droplets enables future selection- or evolution-based studies.
Keywords: Artificial Organelles; Droplet-Based Microfluidics; Nonequilibrium Processes; Phase Transitions.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Active coacervate droplets as a model for membraneless organelles and protocells.Nat Commun. 2020 Oct 14;11(1):5167. doi: 10.1038/s41467-020-18815-9. Nat Commun. 2020. PMID: 33056997 Free PMC article.
-
Dynamics of Synthetic Membraneless Organelles in Microfluidic Droplets.Angew Chem Int Ed Engl. 2019 Oct 7;58(41):14489-14494. doi: 10.1002/anie.201907278. Epub 2019 Sep 3. Angew Chem Int Ed Engl. 2019. PMID: 31334587
-
Self-programmed enzyme phase separation and multiphase coacervate droplet organization.Chem Sci. 2021 Jan 25;12(8):2794-2802. doi: 10.1039/d0sc06418a. Chem Sci. 2021. PMID: 34164043 Free PMC article.
-
Analysis of biomolecular condensates and protein phase separation with microfluidic technology.Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118823. doi: 10.1016/j.bbamcr.2020.118823. Epub 2020 Aug 13. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 32800925 Review.
-
Coacervate Droplets for Synthetic Cells.Small Methods. 2023 Dec;7(12):e2300496. doi: 10.1002/smtd.202300496. Epub 2023 Jul 18. Small Methods. 2023. PMID: 37462244 Review.
Cited by
-
Nonequilibrium Wet-Dry Cycling Acts as a Catalyst for Chemical Reactions.J Phys Chem B. 2024 Feb 22;128(7):1724-1736. doi: 10.1021/acs.jpcb.3c05824. Epub 2024 Feb 9. J Phys Chem B. 2024. PMID: 38335971 Free PMC article.
-
Programmable Enzymatic Reaction Network in Artificial Cell-Like Polymersomes.Adv Sci (Weinh). 2024 Jun;11(24):e2305760. doi: 10.1002/advs.202305760. Epub 2024 Apr 16. Adv Sci (Weinh). 2024. PMID: 38627986 Free PMC article.
-
Phase Transitions in Chemically Fueled, Multiphase Complex Coacervate Droplets.Angew Chem Int Ed Engl. 2022 Nov 14;61(46):e202211905. doi: 10.1002/anie.202211905. Epub 2022 Oct 18. Angew Chem Int Ed Engl. 2022. PMID: 36067054 Free PMC article.
-
Complex Coacervate Materials as Artificial Cells.Acc Mater Res. 2023 Feb 13;4(3):287-298. doi: 10.1021/accountsmr.2c00239. eCollection 2023 Mar 24. Acc Mater Res. 2023. PMID: 37009061 Free PMC article.
-
The critical helping hand of water: theory shows the way to obtain elusive, granular information about kinetic asymmetry driven systems.Chem Sci. 2025 Jul 21;16(33):14940-14955. doi: 10.1039/d5sc03256c. eCollection 2025 Aug 20. Chem Sci. 2025. PMID: 40727837 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

