Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer
- PMID: 35657320
- PMCID: PMC9701133
- DOI: 10.1056/NEJMoa2200075
Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer
Abstract
Background: The role of adjuvant chemotherapy in stage II colon cancer continues to be debated. The presence of circulating tumor DNA (ctDNA) after surgery predicts very poor recurrence-free survival, whereas its absence predicts a low risk of recurrence. The benefit of adjuvant chemotherapy for ctDNA-positive patients is not well understood.
Methods: We conducted a trial to assess whether a ctDNA-guided approach could reduce the use of adjuvant chemotherapy without compromising recurrence risk. Patients with stage II colon cancer were randomly assigned in a 2:1 ratio to have treatment decisions guided by either ctDNA results or standard clinicopathological features. For ctDNA-guided management, a ctDNA-positive result at 4 or 7 weeks after surgery prompted oxaliplatin-based or fluoropyrimidine chemotherapy. Patients who were ctDNA-negative were not treated. The primary efficacy end point was recurrence-free survival at 2 years. A key secondary end point was adjuvant chemotherapy use.
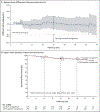
Results: Of the 455 patients who underwent randomization, 302 were assigned to ctDNA-guided management and 153 to standard management. The median follow-up was 37 months. A lower percentage of patients in the ctDNA-guided group than in the standard-management group received adjuvant chemotherapy (15% vs. 28%; relative risk, 1.82; 95% confidence interval [CI], 1.25 to 2.65). In the evaluation of 2-year recurrence-free survival, ctDNA-guided management was noninferior to standard management (93.5% and 92.4%, respectively; absolute difference, 1.1 percentage points; 95% CI, -4.1 to 6.2 [noninferiority margin, -8.5 percentage points]). Three-year recurrence-free survival was 86.4% among ctDNA-positive patients who received adjuvant chemotherapy and 92.5% among ctDNA-negative patients who did not.
Conclusions: A ctDNA-guided approach to the treatment of stage II colon cancer reduced adjuvant chemotherapy use without compromising recurrence-free survival. (Supported by the Australian National Health and Medical Research Council and others; DYNAMIC Australian New Zealand Clinical Trials Registry number, ACTRN12615000381583.).
Copyright © 2022 Massachusetts Medical Society.
Figures


Comment in
-
Liquid Biopsy for Precision Adjuvant Chemotherapy in Colon Cancer.N Engl J Med. 2022 Jun 16;386(24):2330-2331. doi: 10.1056/NEJMe2204625. Epub 2022 Jun 4. N Engl J Med. 2022. PMID: 35657319 No abstract available.
-
ctDNA guides omission of adjuvant chemotherapy for stage II CRC.Nat Rev Clin Oncol. 2022 Aug;19(8):493. doi: 10.1038/s41571-022-00657-7. Nat Rev Clin Oncol. 2022. PMID: 35750857 No abstract available.
-
More Precision in Adjuvant Chemotherapy for Stage II Colon Cancer Using Liquid Biopsy After Surgery.Gastroenterology. 2022 Nov;163(5):1471-1472. doi: 10.1053/j.gastro.2022.07.083. Epub 2022 Aug 10. Gastroenterology. 2022. PMID: 35963370 No abstract available.
-
Circulating Tumor DNA Guiding Adjuvant Therapy in Colon Cancer.N Engl J Med. 2022 Aug 25;387(8):759. doi: 10.1056/NEJMc2209374. N Engl J Med. 2022. PMID: 36001720 No abstract available.
-
Circulating Tumor DNA Guiding Adjuvant Therapy in Colon Cancer.N Engl J Med. 2022 Aug 25;387(8):759-760. doi: 10.1056/NEJMc2209374. N Engl J Med. 2022. PMID: 36001721 No abstract available.
-
ctDNA-guided adjuvant chemotherapy for colorectal cancer-ready for prime time?Cancer Cell. 2022 Sep 12;40(9):911-913. doi: 10.1016/j.ccell.2022.08.017. Epub 2022 Sep 1. Cancer Cell. 2022. PMID: 36055230
-
Circulating tumor DNA analysis: potential to revise adjuvant therapy for stage II colorectal cancer.Signal Transduct Target Ther. 2022 Sep 5;7(1):308. doi: 10.1038/s41392-022-01164-y. Signal Transduct Target Ther. 2022. PMID: 36064813 Free PMC article. No abstract available.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. - PubMed
-
- André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015; 33: 4176–87. - PubMed
-
- Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol 2015; 54:5–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
