A Systematic Review of Pharmacovigilance Systems in Developing Countries Using the WHO Pharmacovigilance Indicators
- PMID: 35657484
- PMCID: PMC9356965
- DOI: 10.1007/s43441-022-00415-y
A Systematic Review of Pharmacovigilance Systems in Developing Countries Using the WHO Pharmacovigilance Indicators
Abstract
Background: In the context of the growth of pharmacovigilance (PV) among developing countries, this systematic review aims to synthesise current research evaluating developing countries' PV systems' performance.
Methods: EMBASE, MEDLINE, CINAHL Plus and Web of Science were searched for peer-reviewed studies published in English between 2012 and 2021. Reference lists of included studies were screened. Included studies were quality assessed using Hawker et al.'s nine-item checklist; data were extracted using the WHO PV indicators checklist. Scores were assigned to each group of indicators and used to compare countries' PV performance.
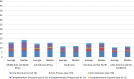
Results: Twenty-one unique studies from 51 countries were included. Of a total possible quality score of 36, most studies were rated medium (n = 7 studies) or high (n = 14 studies). Studies obtained an average score of 17.2 out of a possible 63 of the WHO PV indicators. PV system performance in all 51 countries was low (14.86/63; range: 0-26). Higher average scores were obtained in the 'Core' (9.27/27) compared to 'Complementary' (5.59/36) indicators. Overall performance for 'Process' and 'Outcome' indicators was lower than that of 'Structural'.
Conclusion: This first systematic review of studies evaluating PV performance in developing countries provides an in-depth understanding of factors affecting PV system performance.
Keywords: Benchmarking; Developing countries; Evaluation studies; Pharmacovigilance; Programme evaluation.
© 2022. The Author(s).
Conflict of interest statement
Hamza Y. Garashi is an employee on a PhD scholarship from the Kuwaiti Ministry of Health. Douglas T. Steinke and Ellen I. Schafheutle have no conflict of interest that is directly relevant to the content of this study.
Figures


References
-
- Segura-Bedmar I, Martinez P. Special issue on mining the pharmacovigilance literature. J Biomed Inform. 2014;49:1–2. doi: 10.1016/j.jbi.2014.04.007. - DOI
-
- World Health Organization (WHO). The Importance of Pharmacovigilance: Safety Monitoring of Medicinal Products Geneva: World Health Organization; 2002. https://apps.who.int/iris/handle/10665/42493. Accessed 28 Feb 2022
-
- World Health Organization (WHO). Clinical trials Geneva: World Health Organization; 2022. https://www.who.int/health-topics/clinical-trials#tab=tab_1. Accessed 1 March 2022
-
- NIH National Institute on Aging. What Are Clinical Trials and Studies? Baltimore, MD.: National Institutes of Health (NIH) National Institute on Aging (NIA); 2020. https://www.nia.nih.gov/health/what-are-clinical-trials-and-studies. Accessed 1 March 2022
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

