Mechanical, Material and Morphological Adaptations of Healthy Lower Limb Tendons to Mechanical Loading: A Systematic Review and Meta-Analysis
- PMID: 35657492
- PMCID: PMC9474511
- DOI: 10.1007/s40279-022-01695-y
Mechanical, Material and Morphological Adaptations of Healthy Lower Limb Tendons to Mechanical Loading: A Systematic Review and Meta-Analysis
Abstract
Background: Exposure to increased mechanical loading during physical training can lead to increased tendon stiffness. However, the loading regimen that maximises tendon adaptation and the extent to which adaptation is driven by changes in tendon material properties or tendon geometry is not fully understood.
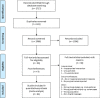
Objective: To determine (1) the effect of mechanical loading on tendon stiffness, modulus and cross-sectional area (CSA); (2) whether adaptations in stiffness are driven primarily by changes in CSA or modulus; (3) the effect of training type and associated loading parameters (relative intensity; localised strain, load duration, load volume and contraction mode) on stiffness, modulus or CSA; and (4) whether the magnitude of adaptation in tendon properties differs between age groups.
Methods: Five databases (PubMed, Scopus, CINAHL, SPORTDiscus, EMBASE) were searched for studies detailing load-induced adaptations in tendon morphological, material or mechanical properties. Standardised mean differences (SMDs) with 95% confidence intervals (CIs) were calculated and data were pooled using a random effects model to estimate variance. Meta regression was used to examine the moderating effects of changes in tendon CSA and modulus on tendon stiffness.
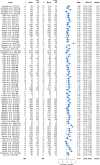
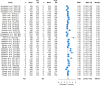
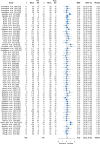
Results: Sixty-one articles met the inclusion criteria. The total number of participants in the included studies was 763. The Achilles tendon (33 studies) and the patella tendon (24 studies) were the most commonly studied regions. Resistance training was the main type of intervention (49 studies). Mechanical loading produced moderate increases in stiffness (standardised mean difference (SMD) 0.74; 95% confidence interval (CI) 0.62-0.86), large increases in modulus (SMD 0.82; 95% CI 0.58-1.07), and small increases in CSA (SMD 0.22; 95% CI 0.12-0.33). Meta-regression revealed that the main moderator of increased stiffness was modulus. Resistance training interventions induced greater increases in modulus than other training types (SMD 0.90; 95% CI 0.65-1.15) and higher strain resistance training protocols induced greater increases in modulus (SMD 0.82; 95% CI 0.44-1.20; p = 0.009) and stiffness (SMD 1.04; 95% CI 0.65-1.43; p = 0.007) than low-strain protocols. The magnitude of stiffness and modulus differences were greater in adult participants.
Conclusions: Mechanical loading leads to positive adaptation in lower limb tendon stiffness, modulus and CSA. Studies to date indicate that the main mechanism of increased tendon stiffness due to physical training is increased tendon modulus, and that resistance training performed at high compared to low localised tendon strains is associated with the greatest positive tendon adaptation. PROSPERO registration no.: CRD42019141299.
© 2022. The Author(s).
Conflict of interest statement
Stephanie Lazarczuk, Nirav Maniar, David Opar, Steven Duhig, Anthony Shield, Rod Barrett and Matthew Bourne declare that they have no conflicts of interest relevant to the content of this review.
Figures

Similar articles
-
Human tendon adaptation in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults.Sports Med Open. 2015 Dec;1(1):7. doi: 10.1186/s40798-015-0009-9. Epub 2015 Mar 27. Sports Med Open. 2015. PMID: 27747846 Free PMC article. Review.
-
Low-load blood flow restriction training induces similar morphological and mechanical Achilles tendon adaptations compared with high-load resistance training.J Appl Physiol (1985). 2019 Dec 1;127(6):1660-1667. doi: 10.1152/japplphysiol.00602.2019. Epub 2019 Nov 14. J Appl Physiol (1985). 2019. PMID: 31725362
-
Effects of high loading by eccentric triceps surae training on Achilles tendon properties in humans.Eur J Appl Physiol. 2018 Aug;118(8):1725-1736. doi: 10.1007/s00421-018-3904-1. Epub 2018 Jun 1. Eur J Appl Physiol. 2018. PMID: 29858689
-
Evidence-Based High-Loading Tendon Exercise for 12 Weeks Leads to Increased Tendon Stiffness and Cross-Sectional Area in Achilles Tendinopathy: A Controlled Clinical Trial.Sports Med Open. 2022 Dec 20;8(1):149. doi: 10.1186/s40798-022-00545-5. Sports Med Open. 2022. PMID: 36538166 Free PMC article.
-
Effect of Strength Training on Biomechanical and Neuromuscular Variables in Distance Runners: A Systematic Review and Meta-Analysis.Sports Med. 2020 Jan;50(1):133-150. doi: 10.1007/s40279-019-01184-9. Sports Med. 2020. PMID: 31541409
Cited by
-
In Vivo Strain Patterns in the Achilles Tendon During Dynamic Activities: A Comprehensive Survey of the Literature.Sports Med Open. 2023 Jul 19;9(1):60. doi: 10.1186/s40798-023-00604-5. Sports Med Open. 2023. PMID: 37466866 Free PMC article. Review.
-
Hydrolysed Collagen Supplementation Enhances Patellar Tendon Adaptations to 12 Weeks' Resistance Training in Middle-Aged Men.Eur J Sport Sci. 2025 Apr;25(4):e12281. doi: 10.1002/ejsc.12281. Eur J Sport Sci. 2025. PMID: 40100255 Free PMC article. Clinical Trial.
-
In Achilles Tendinopathy the Symptomatic Tendon Differs from the Asymptomatic Tendon While Exercise Therapy Has Little Effect on Asymmetries-An Ancillary Analysis of Data from a Controlled Clinical Trial.J Clin Med. 2023 Jan 31;12(3):1102. doi: 10.3390/jcm12031102. J Clin Med. 2023. PMID: 36769750 Free PMC article.
-
Where Does Blood Flow Restriction Fit in the Toolbox of Athletic Development? A Narrative Review of the Proposed Mechanisms and Potential Applications.Sports Med. 2023 Nov;53(11):2077-2093. doi: 10.1007/s40279-023-01900-6. Epub 2023 Aug 14. Sports Med. 2023. PMID: 37578669 Free PMC article. Review.
-
Maximal Lower Limb Strength in Patellar Tendinopathy: A Systematic Review With Meta-Analysis.J Athl Train. 2024 Feb 1;59(2):159-172. doi: 10.4085/1062-6050-0662.22. J Athl Train. 2024. PMID: 37071550 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

