Myocardial mesostructure and mesofunction
- PMID: 35657613
- PMCID: PMC9273275
- DOI: 10.1152/ajpheart.00059.2022
Myocardial mesostructure and mesofunction
Abstract
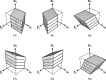
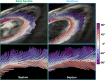
The complex and highly organized structural arrangement of some five billion cardiomyocytes directs the coordinated electrical activity and mechanical contraction of the human heart. The characteristic transmural change in cardiomyocyte orientation underlies base-to-apex shortening, circumferential shortening, and left ventricular torsion during contraction. Individual cardiomyocytes shorten ∼15% and increase in diameter ∼8%. Remarkably, however, the left ventricular wall thickens by up to 30-40%. To accommodate this, the myocardium must undergo significant structural rearrangement during contraction. At the mesoscale, collections of cardiomyocytes are organized into sheetlets, and sheetlet shear is the fundamental mechanism of rearrangement that produces wall thickening. Herein, we review the histological and physiological studies of myocardial mesostructure that have established the sheetlet shear model of wall thickening. Recent developments in tissue clearing techniques allow for imaging of whole hearts at the cellular scale, whereas magnetic resonance imaging (MRI) and computed tomography (CT) can image the myocardium at the mesoscale (100 µm to 1 mm) to resolve cardiomyocyte orientation and organization. Through histology, cardiac diffusion tensor imaging (DTI), and other modalities, mesostructural sheetlets have been confirmed in both animal and human hearts. Recent in vivo cardiac DTI methods have measured reorientation of sheetlets during the cardiac cycle. We also examine the role of pathological cardiac remodeling on sheetlet organization and reorientation, and the impact this has on ventricular function and dysfunction. We also review the unresolved mesostructural questions and challenges that may direct future work in the field.
Keywords: cardiac anatomy; diffusion tensor imaging; mechanics; mesostructured; sheetlets.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
-
- Auchampach J, Han L, Huang GN, Kühn B, Lough JW, O'Meara CC, Payumo AY, Rosenthal NA, Sucov HM, Yutzey KE, Patterson M. Measuring cardiomyocyte cell-cycle activity and proliferation in the age of heart regeneration. Am J Physiol Heart Circ Physiol 322: H579–H596, 2022. doi:10.1152/ajpheart.00666.2021. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

