Validation Parameters of Patient-Generated Data for Digitally Recorded Allergic Rhinitis Symptom and Medication Scores in the @IT.2020 Project: Exploratory Study
- PMID: 35657659
- PMCID: PMC9206201
- DOI: 10.2196/31491
Validation Parameters of Patient-Generated Data for Digitally Recorded Allergic Rhinitis Symptom and Medication Scores in the @IT.2020 Project: Exploratory Study
Abstract
Background: Mobile health technologies enable allergists to monitor disease trends by collecting daily patient-reported outcomes of allergic rhinitis. To this end, patients with allergies are usually required to enter their symptoms and medication repetitively over long time periods, which may present a risk to data completeness and quality in the case of insufficient effort reporting. Completeness of patient's recording is easily measured. In contrast, the intrinsic quality and accuracy of the data entered by the patients are more elusive.
Objective: The aim of this study was to explore the association of adherence to digital symptom recording with a predefined set of parameters of the patient-generated symptom and medication scores and to identify parameters that may serve as proxy measure of the quality and reliability of the information recorded by the patient.
Methods: The @IT.2020 project investigates the diagnostic synergy of mobile health and molecular allergology in patients with seasonal allergic rhinitis. In its pilot phase, 101 children with seasonal allergic rhinitis were recruited in Rome and instructed to record their symptoms, medication intake, and general conditions daily via a mobile app (AllergyMonitor) during the relevant pollen season. We measured adherence to daily recording as the percentage of days with data recording in the observation period. We examined the patient's trajectories of 3 disease indices (Rhinoconjunctivitis Total Symptom Score [RTSS], Combined Symptom and Medication Score [CSMS], and Visual Analogue Scale [VAS]) as putative proxies of data quality with the following 4 parameters: (1) intravariation index, (2) percentage of zero values, (3) coefficient of variation, and (4) percentage of changes in trend. Lastly, we examined the relationship between adherence to recording and each of the 4 proxy measures.
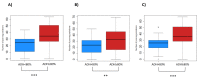
Results: Adherence to recording ranged from 20% (11/56) to 100% (56/56), with 64.4% (65/101) and 35.6% (36/101) of the patients' values above (highly adherent patients) or below (low adherent patients) the threshold of 80%, respectively. The percentage of zero values, the coefficient of variation, and the intravariation index did not significantly change with the adherence to recording. By contrast, the proportion of changes in trend was significantly higher among highly adherent patients, independently from the analyzed score (RTSS, CSMS, and VAS).
Conclusions: The percentage of changes in the trend of RTSS, CSMS, and VAS is a valuable candidate to validate the quality and accuracy of the data recorded by patients with allergic rhinitis during the pollen season. The performance of this parameter must be further investigated in real-life conditions before it can be recommended for routine use in apps and electronic diaries devoted to the management of patients with allergic rhinitis.
Keywords: allergic rhinitis; allergies; allergy monitor; digital health; health applications; mHealth; medication scores; mobile health; patient-generated data; patient-reported outcomes; symptom scores.
©Stephanie Dramburg, Serena Perna, Marco Di Fraia, Salvatore Tripodi, Stefania Arasi, Sveva Castelli, Danilo Villalta, Francesca Buzzulini, Ifigenia Sfika, Valeria Villella, Ekaterina Potapova, Maria Antonia Brighetti, Alessandro Travaglini, Pierluigi Verardo, Simone Pelosi, Paolo Maria Matricardi. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org), 03.06.2022.
Conflict of interest statement
Conflicts of Interest: PMM reports grants and personal fees from TPS Software Production, outside the submitted work. ST is a cofounder of TPS Software Production, which has developed the AllergyMonitor app. S Pelosi is a cofounder of TPS Software Production. All other authors declare no conflicts of interest.
Figures

References
-
- Nahum JL, Fu MR, Scagliola J, Rodorigo M, Tobik S, Guth A, Axelrod D. Real-time electronic patient evaluation of lymphedema symptoms, referral, and satisfaction: a cross-sectional study. Mhealth. 2021;7:20. doi: 10.21037/mhealth-20-118. doi: 10.21037/mhealth-20-118.mh-07-20-118 - DOI - PMC - PubMed
-
- Gomis-Pastor M, Mirabet Perez S, Roig Minguell E, Brossa Loidi V, Lopez Lopez L, Ros Abarca S, Galvez Tugas E, Mas-Malagarriga N, Mangues Bafalluy MA. Mobile Health to Improve Adherence and Patient Experience in Heart Transplantation Recipients: The mHeart Trial. Healthcare (Basel) 2021 Apr 14;9(4):463. doi: 10.3390/healthcare9040463. https://www.mdpi.com/resolver?pii=healthcare9040463 healthcare9040463 - DOI - PMC - PubMed
-
- Tripodi S, Giannone A, Sfika I, Pelosi S, Dramburg S, Bianchi A, Pizzulli A, Florack J, Villella V, Potapova E, Matricardi PM. Digital technologies for an improved management of respiratory allergic diseases: 10 years of clinical studies using an online platform for patients and physicians. Ital J Pediatr. 2020 Jul 25;46(1):105. doi: 10.1186/s13052-020-00870-z. https://ijponline.biomedcentral.com/articles/10.1186/s13052-020-00870-z 10.1186/s13052-020-00870-z - DOI - PMC - PubMed
-
- Khusial RJ, Honkoop PJ, Usmani O, Soares M, Simpson A, Biddiscombe M, Meah S, Bonini M, Lalas A, Polychronidou E, Koopmans JG, Moustakas K, Snoeck-Stroband JB, Ortmann S, Votis K, Tzovaras D, Chung KF, Fowler S, Sont JK, myAirCoach study group Effectiveness of myAirCoach: A mHealth Self-Management System in Asthma. J Allergy Clin Immunol Pract. 2020 Jun;8(6):1972–1979.e8. doi: 10.1016/j.jaip.2020.02.018. https://linkinghub.elsevier.com/retrieve/pii/S2213-2198(20)30178-1 S2213-2198(20)30178-1 - DOI - PubMed
-
- Steel PAD, Bodnar D, Bonito M, Torres-Lavoro J, Eid DB, Jacobowitz A, Shemesh A, Tanouye R, Rumble P, DiCello D, Sharma R, Farmer B, Pomerantz S, Zhang Y. MyEDCare: Evaluation of a Smartphone-Based Emergency Department Discharge Process. Appl Clin Inform. 2021 Mar;12(2):362–371. doi: 10.1055/s-0041-1729165. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

