Nanomolar affinity of EF-hands in neuronal calcium sensor 1 for bivalent cations Pb2+, Mn2+, and Hg2
- PMID: 35657675
- PMCID: PMC9611292
- DOI: 10.1093/mtomcs/mfac039
Nanomolar affinity of EF-hands in neuronal calcium sensor 1 for bivalent cations Pb2+, Mn2+, and Hg2
Abstract
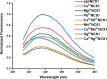
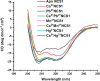
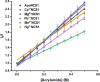
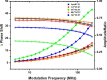
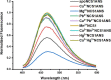
Abiogenic metals Pb and Hg are highly toxic since chronic and/or acute exposure often leads to severe neuropathologies. Mn2+ is an essential metal ion but in excess can impair neuronal function. In this study, we address in vitro the interactions between neuronal calcium sensor 1 (NCS1) and divalent cations. Results showed that non-physiological ions (Pb2+ and Mn2+) bind to EF-hands in NCS1 with nanomolar affinity and lower equilibrium dissociation constant than the physiological Ca2+ ion. (Kd, Pb2+ = 7.0 ± 1.0 nM; Kd, Mn2+ = 34.0 ± 6.0 nM; K). Native ultra-high resolution mass spectrometry (FT-ICR MS) and trapped ion mobility spectrometry-mass spectrometry (nESI-TIMS-MS) studies provided the NCS1-metal complex compositions-up to four Ca2+ or Mn2+ ions and three Pb2+ ions (M⋅Pb1-3Ca1-3, M⋅Mn1-4Ca1-2, and M⋅Ca1-4) were observed in complex-and similarity across the mobility profiles suggests that the overall native structure is preserved regardless of the number and type of cations. However, the non-physiological metal ions (Pb2+, Mn2+, and Hg2+) binding to NCS1 leads to more efficient quenching of Trp emission and a decrease in W30 and W103 solvent exposure compared to the apo and Ca2+ bound form, although the secondary structural rearrangement and exposure of hydrophobic sites are analogous to those for Ca2+ bound protein. Only Pb2+ and Hg2+ binding to EF-hands leads to the NCS1 dimerization whereas Mn2+ bound NCS1 remains in the monomeric form, suggesting that other factors in addition to metal ion coordination, are required for protein dimerization.
Keywords: EF-hands; calcium; fluorescence; metal binding; neuronal calcium sensor 1; non-physiological metals.
© The Author(s) 2022. Published by Oxford University Press.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Ebashi S., Endo M., Calcium and muscle contraction. Prog. Biophys. Mol. Biol., 1968, 18 (9), 123–183. - PubMed
-
- Augustine G. J., Santamaria F., Tanaka K., Local calcium signaling in neurons. Neuron, 2003, 40 (2), 331–346. - PubMed
-
- Stricker S. A., Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol., 1999, 211 (2), 157–176. - PubMed
-
- Mattson M. P., Chan S. L., Calcium orchestrates apoptosis. Nat. Cell Biol., 2003, 5 (12), 1041–1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

