The Caenorhabditis elegans TDRD5/7-like protein, LOTR-1, interacts with the helicase ZNFX-1 to balance epigenetic signals in the germline
- PMID: 35657999
- PMCID: PMC9200344
- DOI: 10.1371/journal.pgen.1010245
The Caenorhabditis elegans TDRD5/7-like protein, LOTR-1, interacts with the helicase ZNFX-1 to balance epigenetic signals in the germline
Abstract
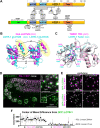
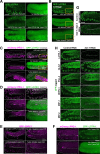
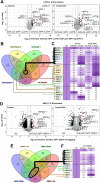
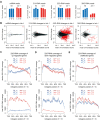
LOTUS and Tudor domain containing proteins have critical roles in the germline. Proteins that contain these domains, such as Tejas/Tapas in Drosophila, help localize the Vasa helicase to the germ granules and facilitate piRNA-mediated transposon silencing. The homologous proteins in mammals, TDRD5 and TDRD7, are required during spermiogenesis. Until now, proteins containing both LOTUS and Tudor domains in Caenorhabditis elegans have remained elusive. Here we describe LOTR-1 (D1081.7), which derives its name from its LOTUS and Tudor domains. Interestingly, LOTR-1 docks next to P granules to colocalize with the broadly conserved Z-granule helicase, ZNFX-1. The Tudor domain of LOTR-1 is required for its Z-granule retention. Like znfx-1 mutants, lotr-1 mutants lose small RNAs from the 3' ends of WAGO and mutator targets, reminiscent of the loss of piRNAs from the 3' ends of piRNA precursor transcripts in mouse Tdrd5 mutants. Our work shows that LOTR-1 acts with ZNFX-1 to bring small RNA amplifying mechanisms towards the 3' ends of its RNA templates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

