An innate IL-25-ILC2-MDSC axis creates a cancer-permissive microenvironment for Apc mutation-driven intestinal tumorigenesis
- PMID: 35658010
- PMCID: PMC7612821
- DOI: 10.1126/sciimmunol.abn0175
An innate IL-25-ILC2-MDSC axis creates a cancer-permissive microenvironment for Apc mutation-driven intestinal tumorigenesis
Abstract
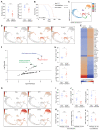
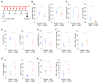
Interleukin-25 (IL-25) and group 2 innate lymphoid cells (ILC2s) defend the host against intestinal helminth infection and are associated with inappropriate allergic reactions. IL-33-activated ILC2s were previously found to augment protective tissue-specific pancreatic cancer immunity. Here, we showed that intestinal IL-25-activated ILC2s created an innate cancer-permissive microenvironment. Colorectal cancer (CRC) patients with higher tumor IL25 expression had reduced survival and increased IL-25R-expressing tumor-resident ILC2s and myeloid-derived suppressor cells (MDSCs) associated with impaired antitumor responses. Ablation of IL-25 signaling reduced tumors, virtually doubling life expectancy in an Apc mutation-driven model of spontaneous intestinal tumorigenesis. Mechanistically, IL-25 promoted intratumoral ILC2s, which sustained tumor-infiltrating MDSCs to suppress antitumor immunity. Therapeutic antibody-mediated blockade of IL-25 signaling decreased intratumoral ILC2s, MDSCs, and adenoma/adenocarcinoma while increasing antitumor adaptive T cell and interferon-γ (IFN-γ)-mediated immunity. Thus, the roles of innate epithelium-derived cytokines IL-25 and IL-33 as well as ILC2s in cancer cannot be generalized. The protumoral nature of the IL-25-ILC2 axis in CRC highlights this pathway as a potential therapeutic target against CRC.
Conflict of interest statement
Figures





Comment in
-
ILC2s-Bipartisan politicians in cancer.Sci Immunol. 2022 Jun 3;7(72):eabq2791. doi: 10.1126/sciimmunol.abq2791. Epub 2022 Jun 3. Sci Immunol. 2022. PMID: 35658014 Free PMC article.
References
-
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie ANJ. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of experimental medicine. 2006;203:1105–1116. - PMC - PubMed
-
- Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. - PMC - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

