Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus)
- PMID: 35658165
- PMCID: PMC9645779
- DOI: 10.1002/14651858.CD004272.pub3
Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus)
Abstract
Background: Several studies have evaluated the clinical effectiveness of endocrine therapy alone in women aged 70 years or over with operable breast cancer and who are fit for surgery.
Objectives: To systematically review the evidence for the clinical effectiveness of surgery (with or without adjuvant endocrine therapy) in comparison to primary endocrine therapy in the treatment of operable breast cancer in women aged 70 years and over, both in terms of local progression and mortality.
Search methods: We conducted an updated search of the Cochrane Breast Cancer Group's Specialised Register (27th March 2013) and new searches of the Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 3), MEDLINE, EMBASE, the World Health Organization's International Clinical Trials Registry Platform (apps.who.int/trialsearch/) and www.
Clinicaltrials: gov, using the search terms 'early breast cancer', 'endocrine therapy', 'psychosocial' or 'surgery'.
Selection criteria: Randomised trials comparing surgery, with or without adjuvant endocrine therapy, to primary endocrine therapy in the management of women aged 70 years or over with early breast cancer and who were fit for surgery.
Data collection and analysis: We assessed studies for eligibility and quality, and two review authors independently extracted data from published trials. We derived hazard ratios for time-to-event outcomes, where possible, and used a fixed-effect model for meta-analysis. We extracted toxicity and quality-of-life data, where present. Where outcome data were not available, we contacted trialists and requested unpublished data.
Main results: We identified seven eligible trials, of which six had published time-to-event data and one was published only in abstract form with no usable data. The quality of the allocation concealment was adequate in three studies and unclear in the remainder. In each case the endocrine therapy used was tamoxifen. Data, based on an estimated 1081 deaths in 1571 women, did not show a statistically significant difference in favour of either surgery or primary endocrine therapy in respect of overall survival. However, there was a statistically significant difference in terms of progression-free survival, which favoured surgery with (474 participants) or without endocrine therapy (164 participants). The hazard ratios (HRs) for overall survival were: HR 0.98 (95% confidence interval (CI) 0.81 to 1.20, P = 0.85; 3 trials, 495 participants) for surgery alone versus primary endocrine therapy; HR 0.86 (95% CI 0.73 to 1.00, P = 0.06; 3 trials, 1076 participants) for surgery plus endocrine therapy versus primary endocrine therapy. The HRs for progression-free survival were: HR 0.55 (95% CI 0.39 to 0.77, P = 0.0006) for surgery alone versus primary endocrine therapy; HR 0.65 (95% CI 0.53 to 0.81, P = 0.0001) for surgery plus endocrine therapy versus primary endocrine therapy (each comparison based on only one trial). Tamoxifen-related adverse effects included hot flushes, skin rash, vaginal discharge, indigestion, breast pain, sleepiness, headache, vertigo, itching, hair loss, cystitis, acute thrombophlebitis, nausea, and indigestion. Surgery-related adverse effects included paraesthesia on the ipsilateral arm and lateral thoracic wall in those who had axillary clearance. One study suggested that those undergoing surgery suffered more psychosocial morbidity at three months post-surgery, although this difference had disappeared by two years.
Authors' conclusions: Primary endocrine therapy should only be offered to women with oestrogen receptor (ER)-positive tumours who are unfit for surgery, at increased risk of serious surgical or anaesthetic complications if subjected to surgery, or who refuse surgery. In a cohort of women with significant co-morbid disease and ER-positive tumours it is possible that primary endocrine therapy may be a superior option to surgery. Trials are needed to evaluate the clinical effectiveness of aromatase inhibitors as primary therapy for an infirm older population with ER-positive tumours.
INTRODUÇÃO: Vários estudos avaliaram a efetividade clínica da terapia endócrina como monoterapia em mulheres com 70 ou mais anos de idade que se apresentam com câncer de mama ressecável.
Objetivos: Revisar de forma sistemática as evidências referente à efetividade clínica da cirurgia (com ou sem terapia endócrina adjuvante) em comparação com terapia endócrina primária no tratamento do câncer de mama ressecável em mulheres com 70 ou mais anos de idade, tanto em termos de progressão local quanto de mortalidade. MÉTODOS DE BUSCA: Nós conduzimos uma busca atualizada no the Cochrane Breast Cancer Group's Specialised Register (27 de Março de 2013, fascículo 3) e, novas buscas na the Cochrane Central Register of Controlled Trials (CENTRAL, 2013, fascículo 3), MEDLINE, EMBASE, the World Health Organization's International Clinical Trials Registry Platform(apps.who.int/trialsearch/)e www.
Clinicaltrials: gov, utilizando os unitermos de pesquisa “early breast cancer”, “endocrine therapy”, “psychosocial” ou “surgery”. CRITÉRIO DE SELEÇÃO: Ensaios clínicos randomizados comparando cirurgia, com ou sem terapia endócrina adjuvante à terapia endócrina primária no manejo de mulheres com 70 anos ou mais de idade com câncer de mama inicial e que eram elegíveis para cirurgia. COLETA DOS DADOS E ANÁLISES: Nós avaliamos a elegibilidade e a qualidade dos estudos. Dois autores da revisão extraíram os dados dos estudos de forma independente. Quando possível, nós derivamos as razões de chances para os desfechos do tipo “time‐to‐event” e utilizamos o modelo de efeito fixo para calcular as metanálises. Nós também extraímos dados referentes à toxicidade e à qualidade de vida, quando informados. Quando os dados referentes ao desfecho não estavam relatados, nós contatamos os autores e solicitamos os dados.
Principais resultados: Nós identificamos sete estudos elegíveis, dos quais seis haviam publicado desfechos do tipo “time‐to‐event” e um foi descrito apenas em um resumo a partir do qual não se pode extrair nenhum dado útil. A qualidade do sigilo da alocação foi adequada em três estudos e incerta nos demais. Em todos os estudos a terapia endócrina utilizada foi o tamoxifeno. Os dados, baseados numa estimativa de 1.081 mortes em 1.571 mulheres, não apresentaram diferença estatisticamente significante em favor da cirurgia ou da terapia endócrina primária em relação a sobrevida global. Todavia, houve uma diferença estatisticamente significante em termos de sobrevida livre de progressão favorecendo o grupo tratado com cirurgia (474 participantes também receberam endocrinoterpia; enquanto 164, apenas cirurgia). As razões de chances (hazard ratios, HR) para sobrevida global foram: HR 0,98 (intervalo de confiança (IC) de 95% 0,81 a 1,20; P = 0,85; três estudos; 495 participantes) para cirurgia isolada contra terapia endócrina primária; HR 0,86 (IC 95% 0,73 a 1,00; P = 0,06; três estudos; 1.076 participantes) para cirurgia associada à endocrinoterapia versus endocrinoterapia primária. As taxas de HR para sobrevida livre de progressão foram: HR 0,55 (IC 95% 0,39 a 0,77; P = 0,0006) para cirurgia isolada contra terapia endócrina primária; HR 0,65 (IC 95% 0,53 a 0,81; P = 0,0001) para cirurgia associada à endocrinoterapia versus endocrinoterapia primária (cada comparação baseada em apenas um estudo). Os eventos adversos relacionados ao tamoxifeno incluíram fogachos, erupção cutânea, corrimento vaginal, dispepsia, dor mamária, insônia, dores de cabeça, vertigem, prurido, perda de cabelo, cistite, tromboflebite aguda e náuseas. Os eventos adversos associados à cirurgia incluíram parestesias no braço ipsilateral e na parede torácica lateral nas pacientes submetidas à esvaziamento axilar. Um estudo sugeriu que aquelas submetidas à cirurgia sofreram mais morbidade psicológica no terceiro mês de seguimento pós‐operatório, porém essa diferença desapareceu após dois anos. CONCLUSÃO DOS AUTORES: A terapia endócrina primária deve apenas ser oferecida para mulheres com tumores que apresentem receptores hormonais (RH) positivos e que não sejam candidatas ao tratamento cirúrgico, ou que apresentem elevado risco de complicações cirúrgicas ou anestésicas se submetidas ao procedimento ou que recusem cirurgia. Em uma coorte de mulheres com comorbidades significativas e tumores RH‐positivos é possível que que a terapia endócrina primária possa ser uma opção superior à cirurgia. Ensaios clínicos são necessários para avaliar a efetividade clínica de inibidores de aromatase como terapia endócrina primária em uma população de pacientes idosas e com comorbidades cujos tumores apresentem receptores hormonais positivos.
Antecedentes: Varios estudios han evaluado la efectividad clínica del tratamiento endocrino solo en mujeres de 70 años o más con cáncer de mama operable y que son adecuadas para cirugía.
Objetivos: Examinar sistemáticamente la evidencia de la efectividad clínica de la cirugía (con o sin tratamiento endocrino adyuvante), en comparación con el tratamiento endocrino primario, para el tratamiento del cáncer de mama operable en mujeres de 70 años o más en cuanto a la progresión local y a la mortalidad. MÉTODOS DE BÚSQUEDA: Se realizó una búsqueda actualizada en el Registro Especializado del Grupo Cochrane de Cáncer de Mama (Cochrane Breast Cancer Group) (27 de marzo 2013) y nuevas búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, 2013, Número 3), MEDLINE, EMBASE, la World Health Organization's International Clinical Trials Registry Platform (apps.who.int/trialsearch/) y www.
Clinicaltrials: gov, mediante los términos de búsqueda "early breast cancer", "endocrine therapy", "psychosocial" o "surgery". CRITERIOS DE SELECCIÓN: Ensayos aleatorizados que compararon la cirugía, con o sin tratamiento endocrino adyuvante, con el tratamiento endocrino primario en el tratamiento de mujeres de 70 años o más con cáncer de mama temprano, y que eran aptas para cirugía. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Se evaluó la elegibilidad y la calidad de los estudios, y dos autores de la revisión de forma independiente extrajeron los datos de los ensayos publicados. Cuando fue posible, se derivaron los cocientes de riesgos instantáneos (CRI) de los resultados del tiempo transcurrido hasta el evento y se utilizó un modelo de efectos fijos para el metanálisis. Se obtuvieron los datos de toxicidad y de calidad de vida cuando se presentaron. En los casos en los que no hubo datos de resultado disponibles, se estableció contacto con los autores de los ensayos y se solicitaron datos no publicados.
Resultados principales: Se identificaron siete ensayos elegibles, de los cuales seis habían publicado datos sobre el tiempo transcurrido hasta el evento y uno se publicó sólo en forma de resumen, sin datos utilizables. La calidad de la ocultación de la asignación fue adecuada en tres estudios e incierta en el resto. En cada caso el tratamiento endocrino utilizado fue el tamoxifeno. Los datos, basados en un cálculo de 1081 muertes en 1571 mujeres, no mostraron diferencias estadísticamente significativas a favor de la cirugía o el tratamiento endocrino primario con respecto a la supervivencia general. Sin embargo, hubo una diferencia estadísticamente significativa en cuanto a la supervivencia sin progresión, que favoreció a la cirugía con (474 participantes) o sin tratamiento endocrino (164 participantes). Los cocientes de riesgos instantáneos (CRI) para la supervivencia global fueron: CRI 0,98 (intervalo de confianza [IC] del 95%: 0,81 a 1,20; p = 0,85; tres ensayos, 495 participantes) para cirugía sola versus tratamiento endocrino primario; CRI 0,86 (IC del 95%: 0,73 a 1,00; p = 0,06; tres ensayos, 1076 participantes) para cirugía más tratamiento endocrino versus tratamiento endocrino primario. Los CRI para la supervivencia libre de progresión fueron: CRI 0,55 (IC del 95%: 0,39 a 0,77; p = 0,0006) para cirugía sola versus tratamiento endocrino primario; CRI 0,65 (IC del 95%: 0,53 a 0,81; p = 0,0001) para cirugía más tratamiento endocrino versus tratamiento endocrino primario (cada comparación basada en un solo ensayo). Los efectos adversos relacionados con el tamoxifeno incluyeron sofocos, erupción cutánea, flujo vaginal, indigestión, dolor mamario, somnolencia, dolor de cabeza, vértigo, picazón, pérdida de cabello, cistitis, tromboflebitis aguda, náuseas e indigestión. Los efectos adversos relacionados con la cirugía incluyeron parestesia en el brazo ipsilateral y en la pared torácica lateral en las pacientes a las que se les realizó vaciamiento axilar. Un estudio indicó que las pacientes sometidas a cirugía presentaron más morbilidad psicosocial a los tres meses después de la cirugía, aunque esta diferencia había desaparecido a los dos años.
Conclusiones de los autores: El tratamiento endocrino primario solo se le debe ofrecer a las mujeres con tumores positivos para los receptores de estrógeno (RE) que no son adecuadas para la cirugía, que tienen un mayor riesgo de complicaciones quirúrgicas o anestésicas graves si se someten a una cirugía, o que rechazan la cirugía. En una cohorte de mujeres con una comorbilidades significativas y tumores positivos para los receptores de estrógeno, es posible que el tratamiento endocrino primario sea una opción superior a la cirugía. Se necesitan ensayos para evaluar la efectividad clínica de los inhibidores de la aromatasa como tratamiento primario para una población de edad avanzada enferma con tumores positivos para los receptores de estrógeno.
Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant Reference Number RP‐PG‐1209‐10071). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Figures
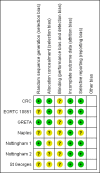

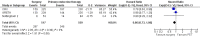
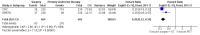



Update of
References
References to studies included in this review
CRC {published and unpublished data}
-
- Bates T, Fennessey M, Riley DL, Baum M, Houghton J, McRae K. Breast cancer in the elderly: surgery improves survival. The results of a cancer research campaign trial. European Journal of Cancer 2001;37(Suppl 5):7.
-
- Bates T, Riley DL, Houghton J, Fallowfield L, Baum M. Breast cancer in elderly women: a Cancer Research Campaign trial comparing treatment with tamoxifen and optimal surgery with tamoxifen alone. British Journal of Surgery 1991;78(5):591‐4. - PubMed
-
- Fallowfield L. Quality of life in the elderly woman with breast cancer treated with tamoxifen and surgery or tamoxifen alone. Journal of Women's Health 1994;3(1):17‐20.
-
- Fennessey M, Bates T, McRae K, Riley D, Houghton J, Baum M. Randomised trial of surgery plus tamoxifen versus tamoxifen‐alone in women over age 70 with operable breast cancer. British Journal of Surgery 2004;91(6):699‐704. - PubMed
-
- Riley D, Bates T, Houghton J. Breast cancer in the elderly patient ‐ the case for clinical trials. The Breast 1995;4:253.
EORTC 10851 {published data only}
-
- Fentiman IS. The nature and treatment of breast cancer in the elderly. Nowotwory 1994;44(Suppl 2):56‐62.
-
- Fentiman IS, Christiaens MR, Paridaens R, Geel A, Rutgers E, Berner J, et al. Treatment of operable breast cancer in the elderly: a randomised clinical trial EORTC 10851 comparing tamoxifen alone with modified radical mastectomy. European Journal of Cancer 2003;39(3):309‐16. - PubMed
GRETA {published data only}
-
- Fabiani E, Giai M, Mustacchi G, Milani S, Sismondi P. Tamoxifen as sole treatment for localised breast cancer of the elderly: preliminary results of the Italian prospective randomized trial. European Journal of Gynaecological Oncology 1991;12 Suppl:59.
-
- Mastacchi G. Hazard ratios and 95% confidence intervals [personal communication]. Email to: G Mustacchi 2005.
-
- Mustacchi G, Ceccherini R, Milani S, Pluchinotta A, Matteis A, Maiorino L, et al. Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: long‐term results of the phase III randomized controlled multicenter GRETA trial. Annals of Oncology 2003;14(3):414‐20. - PubMed
-
- Mustacchi G, Ceccherini R, Pluchinotta A, Matteis A, Maiorino L, Farris A, et al. Results of adjuvant treatment in breast cancer women aged more than 70: Italian cooperative group experience. Tumori 2002;88 Suppl(1):83‐5. - PubMed
-
- Mustacchi G, Mansutti M, Milani S, Pluchinotta A, Farris A, Scanni A, et al. Tamoxifene (tam) and primary breast cancer in old women. European Journal of Cancer 1990;26(2):169.
Naples {published data only}
-
- Capasso I, Nuzzo F, Labonia V, Landi G, Rossi E, Matteis A. Surgery + tamoxifen versus tamoxifen as treatment of stage I and II breast cancer in over to 70 years old women: Ten years follow‐up. Annals of Oncology 2000;11 Suppl(4):20.
-
- Parisi V, Matteis A, Grasso M, Scognamiglio F, Capasso I, Casciello M. Surgery + TAM versus TAM as treatment of stage I and II breast cancer in over 70 years old women. 5th European Conference on Clinical Oncology ECCO5: Federation of European Cancer Societies. 1989.
-
- Thomas R, Capasso I, Matteis A, Labonia V, Landi G, Nuzzo F, et al. Long term survival in elderly breast cancer patients treated with tamoxifen (TAM) alone vs surgery followed by TAM. European Journal of Cancer 1998;34:593.
Nottingham 1 {published data only}
-
- Chakrabarti J, Kenny FS, Syed BM, Robertson JF, Blamey RW, Cheung KL. A randomised trial of mastectomy only versus tamoxifen for treating elderly patients with operable primary breast cancer‐final results at 20‐year follow‐up. Critical Reviews in Oncology/Hematology 2011;78(3):260‐4. - PubMed
-
- Kenny FS, Ellis IO, Elston CW, Robertson JFR, Blamey RW. Long term follow‐up of elderly patients randomized to primary tamoxifen or wedge mastectomy as initial therapy for operable breast cancer. The Breast 1997;6(4):244.
-
- Kenny FS, Robertson JFR, Ellis IO, Elston CW, Blamey RW. Long‐term follow‐up of elderly patients randomized to primary tamoxifen or wedge mastectomy as initial therapy for operable breast cancer. The Breast 1998;7(6):335‐9.
-
- Robertson JF, Ellis IO, Elston CW, Blamey RW. Mastectomy or tamoxifen as initial therapy for operable breast cancer in elderly patients: 5‐year follow‐up. European Journal of Cancer 1992;28A(4‐5):908‐10. - PubMed
Nottingham 2 {published data only}
-
- Cannon PM, Low SC, Dixon A, Ellis IO, Elston CW, Blamey RW. Surgery versus tamoxifen in selected elderly patients with operable breast cancer: early results of a randomised trial. Breast Cancer Research & Treatment 1992;23(1/2):182.
-
- Cannon PM, Low SC, Dixon A, Ellis IO, Elston CW, Blamey RW. Surgery versus tamoxifen in selected elderly patients with operable breast cancer: early results of a randomized trial. Irish Journal of Medical Science 1994;163(2):77.
-
- Willsher PC, Robertson JFR, Jackson L, al Hilaly M, Blamey RW. Investigation of primary tamoxifen therapy for elderly patients with operable breast cancer. The Breast 1997;6:150‐4.
St Georges {published data only}
-
- Bland M. Anonymised IPD from which hazard ratios and 95% confidence intervals were derived [personal communication]. Email to: M Bland 2005.
-
- Gazet JC, Ford HT, Coombes RC, Bland JM, Sutcliffe R, Quilliam J, et al. Prospective randomized trial of tamoxifen vs surgery in elderly patients with breast cancer. European Journal of Surgical Oncology 1994;20(3):207‐14. - PubMed
-
- Gazet JC, Markopoulos C, Ford HT, Coombes RC, Bland JM, Dixon RC. A prospective randomized trial of tamoxifen versus surgery in elderly patients with breast cancer ‐ a preliminary report. 1st European Congress on Senology; 27‐30 Mar 1988; Athens. 1988:140. - PubMed
-
- Gazet JC, Markopoulos C, Ford HT, Coombes RC, Bland JM, Dixon RC. Prospective randomised trial of tamoxifen versus surgery in elderly patients with breast cancer. Lancet 1988;1(8587):679‐81. - PubMed
-
- Gazet JC, Sutcliffe R. A randomised trial comparing tamoxifen vs. surgery in patients over the age of 70 with operable breast cancer‐‐final results after 28 years of follow‐up. European Journal of Surgical Oncology 2011;37(9):754‐7. - PubMed
Additional references
ATAC 2005
-
- The ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365:60–2. - PubMed
Bayer 2000
BCCOM 2007
-
- West Midlands Cancer Intelligenve Unit. Breast Cancer Clinical Outcome Measures (BCCOM) Project: Analysis of the management of symptomatic breast cancers diagnosed in 2004. www.wmciu.nhs.uk/documents/BCCOM%20Year%203%20report.pdf (accessed 10th April 2014).
Bland 2005 [pers comm]
-
- Bland M. Anonymised IPD from which hazard ratios and 95% confidence intervals were derived [personal communication]. Email to: M Bland 2005.
Bouchardy 2003
-
- Bouchardy C, Rapiti E, Fioretta G, Laissue P, Neyroud‐Caspar I, Schäfer P, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. Journal of Clinical Oncology 2003;21(19):3580‐7. - PubMed
Bradbeer 1983
-
- Bradbeer JW, Kyngdon J. Primary treatment of breast cancer in elderly women with tamoxifen. Clinical Oncology 1983;9(1):31‐4. - PubMed
Bugeja 1997
Burak 2002
-
- Burak WE, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC. Sentinel lymph node biopsy results in less post‐operative morbidity compared with axillary lymph node dissection for breast cancer. American Journal of Surgery 2002;183(1):23‐7. - PubMed
Chakrabarti 2011
-
- Chakrabarti J, Kenny FS, Syed BM, Robertson JF, Blamey RW, Cheung KL. A randomised trial of mastectomy only versus tamoxifen for treating elderly patients with operable primary breast cancer‐final results at 20‐year follow‐up. Critical Reviews in Oncology/Hematology 2011;78(3):260‐4. - PubMed
Craft 2000
-
- Craft PS, Zhang Y, Brogan J, Tait N, Buckingham JM. Implementing clinical practice guidelines: a community based audit of breast cancer treatment. Australian Capital Territory and South Eastern New South Wales Breast Cancer Treatment Group. Medical Journal of Australia 2000;172(5):213‐6. - PubMed
Crivellari 1991
-
- Crivellari D, Galligioni E, Foladore S, Errante D, Conte G, Nascimben O, et al. Treatment patterns in elderly patients (greater‐than‐or‐equal‐to 70 years) with breast‐carcinoma ‐ a retrospective study of the Gruppo‐Oncologico‐Clinico‐Cooperativo‐Del‐Nord‐Est (GOCCNE). Tumori 1991;77(2):136‐40. - PubMed
Diab 2000
-
- Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women With breast cancer. Journal of the National Cancer Institute 2000;92(7):550‐6. - PubMed
Eiermann 2001
-
- Eiermann W, Paepke S, Appfelstaedt J, Llombart‐Cussac A, Eremin J, Vinholes J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double‐blind multicenter study. Annals of Oncology 2001;12(11):1527‐32. - PubMed
Ellis 2011
-
- Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrazole, and exemestane for postmenopausal women with estrogen receptor‐rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value to the baseline PAM50‐based intrinsic subtype – ACOSOG Z1031. Journal of Clinical Oncology 2011;29(17):2342‐9. - PMC - PubMed
Exterman 2000
-
- Exterman M, Balducci L, Lyman GH. What threshold for adjuvant therapy in older breast cancer patients?. Journal of Clinical Oncology 2000;18(8):1709‐17. - PubMed
Fallowfield 1994
-
- Fallowfield L. Quality of life in the elderly woman with breast cancer treated with tamoxifen and surgery or tamoxifen alone. Journal of Women's Health 1994;3(1):17‐20.
Fennessey 2004
-
- Fennessey M, Bates T, McRae K, Riley D, Houghton J, Baum M. Randomised trial of surgery plus tamoxifen versus tamoxifen‐alone in women over age 70 with operable breast cancer. British Journal of Surgery 2004;91(6):699‐704. - PubMed
Fentiman 2003
-
- Fentiman IS, Christiaens MR, Paridaens R, Geel A, Rutgers E, Berner J, et al. Treatment of operable breast cancer in the elderly: a randomised clinical trial EORTC 10851 comparing tamoxifen alone with modified radical mastectomy. European Journal of Cancer 2003;39(3):309‐16. - PubMed
Garbay 1998
-
- Garbay JR, Bertheault‐Cvitkovic F, Cohen‐Solal Le Nir C, Stevens D, Cherel P, Berlie J, et al. Treatment of breast cancer after 70 years of age. Report of 1143 cases. Chirurgie 2002;123(4):379‐85. - PubMed
Gaskell 1989
-
- Gaskell DJ, Sangster K, Hawkins RA, Chetty U, Forrest APM. Relation between immunocytochemical estimation of oestrogen receptor in elderly patients with primary breast cancer and response to tamoxifen. Lancet 1989;1(8646):1044‐6. - PubMed
Gaskell 1992
-
- Gaskell DJ, Hawkins RA, Carteret S, Chetty U, Sangster K, Forrest APM. Indications for primary tamoxifen in elderly women with breast cancer. British Journal of Surgery 1992;79(12):1317‐20. - PubMed
Gazet 1994
-
- Gazet JC, Ford HT, Coombes RC, Bland JM, Sutcliffe R, Quilliam J, et al. Prospective randomized trial of tamoxifen vs surgery in elderly patients with breast cancer. European Journal of Surgical Oncology 1994;20(3):207‐14. - PubMed
Gazet 2011
-
- Gazet JC, Sutcliffe R. A randomised trial comparing tamoxifen vs. surgery in patients over the age of 70 with operable breast cancer‐‐final results after 28 years of follow‐up. European Journal of Surgical Oncology 2011;37(9):754‐7. - PubMed
Globocan 2010
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010; Vol. 127, issue 12:2893–917. - PubMed
Goldberg 1970
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. ..
Hooper 2002
-
- Hooper SB, Hill AD, Kennedy S, Dijkstra B, Kelly LM, McDermott EW, et al. Tamoxifen as the primary treatment in elderly patients with breast cancer. Irish Journal of Medical Science 2002;171(1):28‐30. - PubMed
Husain 2008
-
- Husain LS, Collins K, Reed M, Wyld L. Choices in cancer treatment: a qualitative study of the older women's (>70 years) perspective. Psycho‐oncology 2008;17(4):410‐6. - PubMed
Kenny 1998
-
- Kenny FS, Robertson JFR, Ellis IO, Elston CW, Blamey RW. Long term follow‐up of elderly patients randomised to primary tamoxifen or wedge mastectomy as initial therapy for operable breast cancer. The Breast 1998;7:335‐9.
Kesseler 1978
-
- Kesseler HJ, Seton JZ. The treatment of operable breast cancer in the elderly female. American Journal of Surgery 1978;135(5):664‐6. - PubMed
McCarty 1983
Monypenny 2003
-
- Monypenny I on behalf of the BCCOM steering group. Breast Cancer Clinical Outcome Measures (BCCOM) Project. Analysis of the management of symptomatic breast cancers diagnosed in 2002. West Midlands Cancer Intelligence Unit 2006.
Mouridsen 2003
-
- Mouridsen H, Gershanovich M, Sun Y, Perez‐Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first‐line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. Journal of Clinical Oncology 2003;21(11):2101‐9. - PubMed
Mustacchi 1998
-
- Mustacchi G, Latteier J, Milani S, Bates T, Houghton J. Tamoxifen versus surgery plus tamoxifen as primary treatment for elderly patients with breast cancer: combined data from the "GRETA" and "CRC" trials. Proceedings of ASCO. 1998; Vol. 17:99a. [Abstract 383]
Mustacchi 2003
-
- Mustacchi G, Ceccherini R, Milani S, Pluchinotta A, Matteis A, Maiorino L, et al. Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: long‐term results of the phase III randomized controlled multicenter GRETA trial. Annals of Oncology 2003;14(3):414‐20. - PubMed
Mustacchi 2005 [pers comm]
-
- Mastacchi G. Hazard ratios and 95% confidence intervals [personal communication]. Email to: G Mustacchi 2005.
NHSIC 2011
-
- NHS Information Centre. National Mastectomy and Breast Reconstruction Audit. www.hscic.gov.uk/catalogue/PUB02731/clin‐audi‐supp‐prog‐mast‐brea‐reco‐2... (Accessed 10th April 2014).
NICE 2002
-
- National Institute for Clinical Excellence (NICE). Guidance on Cancer Services: Improving Outcomes in Breast Cancer. Manual update. London: NICE, 2002.
Oakley 1996
-
- Oakley N, Dennison AR, Shorthouse AJ. A prospective audit of simple mastectomy under local anaesthesia. European Journal of Surgical Oncology 1996;22(2):134–6. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Preece 1982
Reed 2009
-
- Reed MW, Wyld L, Ellis P, Bliss J, Leonard R. Breast cancer in older women: trials and tribulations. Clinical Oncology (Royal College of Radiologists) 2009;21(2):99‐102. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sader 1999
-
- Sader C, Ingram D, Hastrich D. Management of breast cancer in the elderly by complete local excision and tamoxifen alone. Australia and New Zealand Journal of Surgery 1999;69(11):790‐3. - PubMed
Satariano 1994
-
- Satariano WA, Ragland DR. The effect of comorbidity on 3‐year survival of women with primary breast cancer. Annals of Internal Medicine 1994;120(2):104‐10. - PubMed
Silliman 1993
-
- Silliman RA, Balducci L, Goodwin JS, Holmes FF, Leventhal EA. Breast cancer care in old age: What we know, don't know and do. Journal of the National Cancer Institute 1993;85(3):190‐9. - PubMed
Smitt 1995
-
- Smitt MC, Nowels KW, Zdeblick MJ, Jeffrey S, Carlson RW, Stockdale RW, et al. The importance of the lumpectomy surgical margin status in long term results of breast conservation. Cancer 1995;76(2):259‐67. - PubMed
UICC 2009
-
- Union Internationale Contre le Cancer. Breast tumours. In: Sobin LH, Gospodarowicz MK, Wittekind C editor(s). The TNM Classification of Malignant Tumours. 7th Edition. Oxford: Wiley‐Blackwell, 2009:181‐193.
Van Dalsen 1995
-
- Dalsen A, Vries J. Treatment of breast cancer in elderly patients. Journal of Surgical Oncology 1995;60(2):80‐2. - PubMed
Wanebo 1997
Willsher 1997
-
- Willsher PC, Robertson JFR, Jackson L, al Hilaly M, Blamey RW. Investigation of primary tamoxifen therapy for elderly patients with operable breast cancer. The Breast 1997;6:150‐4.
Wyld 2003
-
- Wyld L, Reed MW. The need for targeted research into breast cancer in the elderly. British Journal of Surgery 2003;90(4):388‐99. - PubMed
Wyld 2004
Yusuf 1985
-
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐371. - PubMed
References to other published versions of this review
Publication types
LinkOut - more resources
Full Text Sources

