Concept of Embedded Dipoles as a Versatile Tool for Surface Engineering
- PMID: 35658405
- PMCID: PMC9260959
- DOI: 10.1021/acs.accounts.2c00173
Concept of Embedded Dipoles as a Versatile Tool for Surface Engineering
Abstract
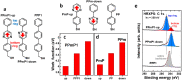
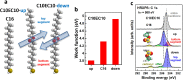
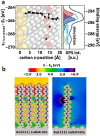
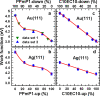
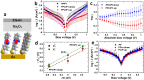
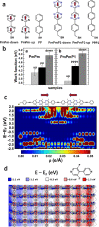
Controlling the physical and chemical properties of surfaces and interfaces is of fundamental relevance in various areas of physical chemistry and a key issue of modern nanotechnology. A highly promising strategy for achieving that control is the use of self-assembled monolayers (SAMs), which are ordered arrays of rodlike molecules bound to the substrate by a suitable anchoring group and carrying a functional tail group at the other end of the molecular backbone. Besides various other applications, SAMs are frequently used in organic electronics for the electrostatic engineering of interfaces by controlling the interfacial level alignment. This is usually achieved by introducing a dipolar tail group at the SAM-semiconductor interface. Such an approach, however, also changes the chemical character of that interface, for example, affecting the growth of subsequent layers. A strategy for avoiding this complication is to embed polar groups into the backbones of the SAM-forming molecules. This allows disentangling electronic interface engineering and the nucleation of further layers, such that both can be optimized independently. This novel concept was successfully demonstrated for both aliphatic and aromatic SAMs on different application-relevant substrates, such as gold, silver, and indium tin oxide. Embedding, for example, ester and pyrimidine groups in different orientations into the backbones of the SAM-forming molecules results in significant work-function changes. These can then be fine-tuned over a wide energy range by growing mixed monolayers consisting of molecules with oppositely oriented polar groups. In such systems, the variation of the work function is accompanied by pronounced shifts of the peaks in X-ray photoelectron spectra, which demonstrates that electrostatically triggered core-level shifts can be as important as the well-established chemical shifts. This illustrates the potential of X-ray photoelectron spectroscopy (XPS) as a tool for probing the local electrostatic energy within monolayers and, in systems like the ones studied here, makes XPS a powerful tool for studying the composition and morphology of binary SAMs. All these experimental observations can be rationalized through simulations, which show that the assemblies of embedded dipolar groups introduce a potential discontinuity within the monolayer, shifting the energy levels above and below the dipoles relative to each other. In molecular and monolayer electronics, embedded-dipole SAMs can be used to control transition voltages and current rectification. In devices based on organic and 2D semiconductors, such as MoS2, they can reduce contact resistances by several orders of magnitude without adversely affecting film growth even on flexible substrates. By varying the orientation of the embedded dipolar moieties, it is also possible to build p- and n-type organic transistors using the same electrode materials (Au). The extensions of the embedded-dipole concept from hybrid interfaces to systems such as metal-organic frameworks is currently underway, which further underlines the high potential of this approach.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Abu-Husein T.; Schuster S.; Egger D. A.; Kind M.; Santowski T.; Wiesner A.; Chiechi R.; Zojer E.; Terfort A.; Zharnikov M. The Effects of Embedded Dipoles in Aromatic Self-Assembled Monolayers. Adv. Funct. Mater. 2015, 25, 3943–3957. 10.1002/adfm.201500899. - DOI
-
- Petritz A.; Krammer M.; Sauter E.; Gärtner M.; Nascimbeni G.; Schrode B.; Fian A.; Gold H.; Cojocaru A.; Karner-Petritz E.; Resel R.; Terfort A.; Zojer E.; Zharnikov M.; Zojer K.; Stadlober B. Embedded Dipole Self-Assembled Monolayers for Contact Resistance Tuning in p- and n-Type Organic Thin Film Transistors and Flexible Electronic Circuits. Adv. Funct. Mater. 2018, 28, 1804462.10.1002/adfm.201804462. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

