A Novel NAMPT Inhibitor-Based Antibody-Drug Conjugate Payload Class for Cancer Therapy
- PMID: 35658441
- PMCID: PMC9204702
- DOI: 10.1021/acs.bioconjchem.2c00178
A Novel NAMPT Inhibitor-Based Antibody-Drug Conjugate Payload Class for Cancer Therapy
Abstract
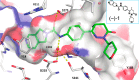
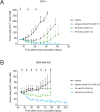
Inhibition of intracellular nicotinamide phosphoribosyltransferase (NAMPT) represents a new mode of action for cancer-targeting antibody-drug conjugates (ADCs) with activity also in slowly proliferating cells. To extend the repertoire of available effector chemistries, we have developed a novel structural class of NAMPT inhibitors as ADC payloads. A structure-activity relationship-driven approach supported by protein structural information was pursued to identify a suitable attachment point for the linker to connect the NAMPT inhibitor with the antibody. Optimization of scaffolds and linker structures led to highly potent effector chemistries which were conjugated to antibodies targeting C4.4a (LYPD3), HER2 (c-erbB2), or B7H3 (CD276) and tested on antigen-positive and -negative cancer cell lines. Pharmacokinetic studies, including metabolite profiling, were performed to optimize the stability and selectivity of the ADCs and to evaluate potential bystander effects. Optimized NAMPTi-ADCs demonstrated potent in vivo antitumor efficacy in target antigen-expressing xenograft mouse models. This led to the development of highly potent NAMPT inhibitor ADCs with a very good selectivity profile compared with the corresponding isotype control ADCs. Moreover, we demonstrate─to our knowledge for the first time─the generation of NAMPTi payload metabolites from the NAMPTi-ADCs in vitro and in vivo. In conclusion, NAMPTi-ADCs represent an attractive new payload class designed for use in ADCs for the treatment of solid and hematological cancers.
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors are current or former employees of Bayer AG. NB, MB, ME, SH, SB, JG, CM, HJ, NBa, UB, RCH, JW, CFN, HW, and AS are stockholders of Bayer AG. All authors (except RCH, UE, JW, DM, CFN, and HW) hold patents connected to this work (WO2019/149637A1, WO2021/013693A1).
Figures

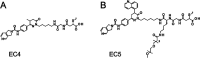
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

