Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up
- PMID: 35658469
- PMCID: PMC9937098
- DOI: 10.1200/JCO.22.00842
Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up
Abstract
Purpose: CARTITUDE-1, a phase Ib/II study evaluating the safety and efficacy of ciltacabtagene autoleucel (cilta-cel) in heavily pretreated patients with relapsed/refractory multiple myeloma, yielded early, deep, and durable responses at 12 months. Here, we present updated results 2 years after last patient in (median follow-up [MFU] approximately 28 months), including analyses of high-risk patient subgroups.
Methods: Eligible patients had relapsed/refractory multiple myeloma, had received ≥ 3 prior lines of therapy or were double refractory to a proteasome inhibitor and immunomodulatory drug and had received prior proteasome inhibitor, immunomodulatory drug, and anti-CD38 therapy. Patients received a single cilta-cel infusion 5-7 days after lymphodepletion. Responses were assessed by an independent review committee.
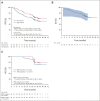
Results: At a MFU of 27.7 months (N = 97), the overall response rate was 97.9% (95% CI, 92.7 to 99.7); 82.5% (95% CI, 73.4 to 89.4) of patients achieved a stringent complete response. Median duration of response was not estimable. Median progression-free survival (PFS) and overall survival (OS) were not reached; 27-month PFS and OS rates were 54.9% (95% CI, 44.0 to 64.6) and 70.4% (95% CI, 60.1 to 78.6), respectively. Overall response rates were high across all subgroups (95.1%-100%). Duration of response, PFS, and/or OS were shorter in patients with high-risk cytogenetics, International Staging System stage III, high tumor burden, or plasmacytomas. The safety profile was manageable with no new cilta-cel-related cytokine release syndrome and one new case of parkinsonism (day 914 after cilta-cel) since the last report.
Conclusion: At approximately 28 months MFU, patients treated with cilta-cel maintained deep and durable responses, observed in both standard and high-risk subgroups. The risk/benefit profile of cilta-cel remained favorable with longer follow-up.
Trial registration: ClinicalTrials.gov NCT03548207.
Figures


References
-
- Kumar SK, Callander NS, Adekola K, et al. : Multiple myeloma, version 7.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:484-493, 2021 - PubMed
-
- Karyopharm Therapeutics, Inc: XPOVIO® (selinexor) prescribing information. https://www.karyopharm.com/wp-content/uploads/2019/07/NDA-212306-SN-0071...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

