A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45)
- PMID: 35658487
- PMCID: PMC9746782
- DOI: 10.1200/JCO.22.01003
A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45)
Abstract
Purpose: ATHENA (ClinicalTrials.gov identifier: NCT03522246) was designed to evaluate rucaparib first-line maintenance treatment in a broad patient population, including those without BRCA1 or BRCA2 (BRCA) mutations or other evidence of homologous recombination deficiency (HRD), or high-risk clinical characteristics such as residual disease. We report the results from the ATHENA-MONO comparison of rucaparib versus placebo.
Methods: Patients with stage III-IV high-grade ovarian cancer undergoing surgical cytoreduction (R0/complete resection permitted) and responding to first-line platinum-doublet chemotherapy were randomly assigned 4:1 to oral rucaparib 600 mg twice a day or placebo. Stratification factors were HRD test status, residual disease after chemotherapy, and timing of surgery. The primary end point of investigator-assessed progression-free survival was assessed in a step-down procedure, first in the HRD population (BRCA-mutant or BRCA wild-type/loss of heterozygosity high tumor), and then in the intent-to-treat population.
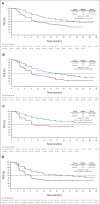
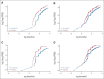
Results: As of March 23, 2022 (data cutoff), 427 and 111 patients were randomly assigned to rucaparib or placebo, respectively (HRD population: 185 v 49). Median progression-free survival (95% CI) was 28.7 months (23.0 to not reached) with rucaparib versus 11.3 months (9.1 to 22.1) with placebo in the HRD population (log-rank P = .0004; hazard ratio [HR], 0.47; 95% CI, 0.31 to 0.72); 20.2 months (15.2 to 24.7) versus 9.2 months (8.3 to 12.2) in the intent-to-treat population (log-rank P < .0001; HR, 0.52; 95% CI, 0.40 to 0.68); and 12.1 months (11.1 to 17.7) versus 9.1 months (4.0 to 12.2) in the HRD-negative population (HR, 0.65; 95% CI, 0.45 to 0.95). The most common grade ≥ 3 treatment-emergent adverse events were anemia (rucaparib, 28.7% v placebo, 0%) and neutropenia (14.6% v 0.9%).
Conclusion: Rucaparib monotherapy is effective as first-line maintenance, conferring significant benefit versus placebo in patients with advanced ovarian cancer with and without HRD.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures







Comment in
-
Rucaparib Maintenance in Upfront Ovarian Cancer: The Long-Lasting Challenge of Predicting Response to Poly (ADP-ribose) Polymerase Inhibitors.J Clin Oncol. 2023 Feb 1;41(4):935-936. doi: 10.1200/JCO.22.01585. Epub 2022 Oct 6. J Clin Oncol. 2023. PMID: 36201722 No abstract available.
-
PARP inhibitors, use early in the first-line maintenance therapy setting but with caution.Ann Transl Med. 2023 Mar 31;11(6):272. doi: 10.21037/atm-2022-81. Epub 2023 Jan 19. Ann Transl Med. 2023. PMID: 37082675 Free PMC article. No abstract available.
References
-
- Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 - PubMed
-
- Gonzalez-Martin A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 - PubMed
-
- Ray-Coquard I, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 - PubMed
-
- Banerjee S, Moore KN, Colombo N, et al. : Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 22:1721-1731, 2021 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

