Contemporary Approach to Acute Myeloid Leukemia Therapy in 2022
- PMID: 35658497
- PMCID: PMC12105117
- DOI: 10.1200/EDBK_349605
Contemporary Approach to Acute Myeloid Leukemia Therapy in 2022
Abstract
Recent advances in acute myeloid leukemia biology and drug development have transformed the therapeutic landscape for patients diagnosed with this disease. By harnessing insights from the study of the molecular pathogenesis of the disease, the acute myeloid leukemia treatment armamentarium now extends beyond conventional cytotoxic agents to include targeted therapies, and immunotherapeutics, with multiple novel modalities under investigation. During the past 5 years, recent drug approvals have also focused attention on disease scenarios and patient populations for whom newer therapies might be deployed. In this review, we highlight select acute myeloid leukemia therapies in the frontline setting through the lens of both disease and patient-related factors. Particular emphasis is placed on the assessment of patient fitness, as contemporary acute myeloid leukemia therapy decisions largely hinge on the determination of whether intensive chemotherapy is suitable for a patient. Additionally, we detail scenarios and areas of controversy wherein disease biology may inspire a reframing of traditional intensive treatment philosophies, regardless of patient fitness. Lastly, we provide an overview of emerging agents that are being investigated in the relapsed/refractory setting.
Conflict of interest statement
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Disclosures provided by the authors and data availability statement (if applicable) are available with this article at DOI
Figures
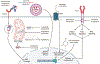
References
-
- Alibhai SM, Leach M, Minden MD, et al. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115:2903–2911. - PubMed
-
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. - PubMed
-
- Østgård LS, Nørgaard JM, Sengeløv H, et al. Comorbidity and performance status in acute myeloid leukemia patients: a nation-wide population-based cohort study. Leukemia. 2015;29:548–555. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

