Genomic Analyses Identify Manganese Homeostasis as a Driver of Group B Streptococcal Vaginal Colonization
- PMID: 35658538
- PMCID: PMC9239048
- DOI: 10.1128/mbio.00985-22
Genomic Analyses Identify Manganese Homeostasis as a Driver of Group B Streptococcal Vaginal Colonization
Abstract
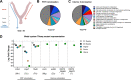
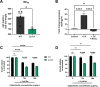
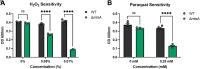
Group B Streptococcus (GBS) is associated with severe infections in utero and in newborn populations, including pneumonia, sepsis, and meningitis. GBS vaginal colonization of the pregnant mother is an important prerequisite for transmission to the newborn and the development of neonatal invasive disease; however, our understanding of the factors required for GBS persistence and ascension in the female reproductive tract (FRT) remains limited. Here, we utilized a GBS mariner transposon (Krmit) mutant library previously developed by our group and identified underrepresented mutations in 535 genes that contribute to survival within the vaginal lumen and colonization of vaginal, cervical, and uterine tissues. From these mutants, we identified 47 genes that were underrepresented in all samples collected, including mtsA, a component of the mtsABC locus, encoding a putative manganese (Mn2+)-dependent ATP-binding cassette transporter. RNA sequencing analysis of GBS recovered from the vaginal tract also revealed a robust increase of mtsA expression during vaginal colonization. We engineered an ΔmtsA mutant strain and found by using inductively coupled plasma mass spectrometry that it exhibited decreased concentrations of intracellular Mn2+, confirming its involvement in Mn2+ acquisition. The ΔmtsA mutant was significantly more susceptible to the metal chelator calprotectin and to oxidative stressors, including both H2O2 and paraquat, than wild-type (WT) GBS. We further observed that the ΔmtsA mutant strain exhibited a significant fitness defect in comparison to WT GBS in vivo by using a murine model of vaginal colonization. Taken together, these data suggest that Mn2+ homeostasis is an important process contributing to GBS survival in the FRT. IMPORTANCE Morbidity and mortality associated with GBS begin with colonization of the female reproductive tract (FRT). To date, our understanding of the factors required for GBS persistence in this environment remain limited. We identified several necessary systems for initial colonization of the vaginal lumen and penetration into the reproductive tissues via transposon mutagenesis sequencing. We determined that mutations in mtsA, the gene encoding a protein putatively involved in manganese (Mn2+) transport, were significantly underrepresented in all in vivo samples collected. We also show that mtsA contributes to Mn2+ acquisition and GBS survival during metal limitation by calprotectin, a metal-chelating protein complex. We further demonstrate that a mutant lacking mtsA is hypersusceptible to oxidative stress induced by both H2O2 and paraquat and has a severe fitness defect compared to WT GBS in the murine vaginal tract. This work reveals the importance of Mn2+ homeostasis at the host-pathogen interface in the FRT.
Keywords: Group B Streptococcus; intrauterine infection; manganese; vaginal colonization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Identification of Zinc-Dependent Mechanisms Used by Group B Streptococcus To Overcome Calprotectin-Mediated Stress.mBio. 2020 Nov 10;11(6):e02302-20. doi: 10.1128/mBio.02302-20. mBio. 2020. PMID: 33173000 Free PMC article.
-
The Group B Streptococcal Adhesin BspC Interacts with Host Cytokeratin 19 To Promote Colonization of the Female Reproductive Tract.mBio. 2022 Oct 26;13(5):e0178122. doi: 10.1128/mbio.01781-22. Epub 2022 Sep 7. mBio. 2022. PMID: 36069447 Free PMC article.
-
Role of MUC5B during Group B Streptococcal Vaginal Colonization.mBio. 2022 Apr 26;13(2):e0003922. doi: 10.1128/mbio.00039-22. Epub 2022 Mar 24. mBio. 2022. PMID: 35323039 Free PMC article.
-
The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen.J Mol Biol. 2019 Jul 26;431(16):2914-2931. doi: 10.1016/j.jmb.2019.01.035. Epub 2019 Jan 31. J Mol Biol. 2019. PMID: 30711542 Free PMC article. Review.
-
Group B streptococcal infection of the genitourinary tract in pregnant and non-pregnant patients with diabetes mellitus: An immunocompromised host or something more?Am J Reprod Immunol. 2021 Dec;86(6):e13501. doi: 10.1111/aji.13501. Epub 2021 Oct 19. Am J Reprod Immunol. 2021. PMID: 34570418 Free PMC article. Review.
Cited by
-
Identification of Glyoxalase A in Group B Streptococcus and its contribution to methylglyoxal tolerance and virulence.bioRxiv [Preprint]. 2024 Dec 19:2024.07.30.605887. doi: 10.1101/2024.07.30.605887. bioRxiv. 2024. Update in: Infect Immun. 2025 Apr 08;93(4):e0054024. doi: 10.1128/iai.00540-24. PMID: 39131367 Free PMC article. Updated. Preprint.
-
A Novel Conserved Protein in Streptococcus agalactiae, BvaP, Is Important for Vaginal Colonization and Biofilm Formation.mSphere. 2022 Dec 21;7(6):e0042122. doi: 10.1128/msphere.00421-22. Epub 2022 Oct 11. mSphere. 2022. PMID: 36218343 Free PMC article.
-
Manganese uptake by MtsABC contributes to the pathogenesis of human pathogen group A streptococcus by resisting host nutritional immune defenses.Infect Immun. 2024 Jul 11;92(7):e0007724. doi: 10.1128/iai.00077-24. Epub 2024 Jun 13. Infect Immun. 2024. PMID: 38869295 Free PMC article.
-
Heterogeneity of the group B streptococcal type VII secretion system and influence on colonization of the female genital tract.bioRxiv [Preprint]. 2023 Jan 25:2023.01.25.525443. doi: 10.1101/2023.01.25.525443. bioRxiv. 2023. Update in: Mol Microbiol. 2023 Aug;120(2):258-275. doi: 10.1111/mmi.15115. PMID: 36747681 Free PMC article. Updated. Preprint.
-
Group B Streptococcus Cas9 variants provide insight into programmable gene repression and CRISPR-Cas transcriptional effects.Commun Biol. 2023 Jun 9;6(1):620. doi: 10.1038/s42003-023-04994-w. Commun Biol. 2023. PMID: 37296208 Free PMC article.
References
-
- Martin JA, Hamilton BE, Osterman MJ. 2018. Births in the United States. NCHS Data Brief no. 318. National Center for Health Statistics, Hyattsville, MD.
-
- Hoyert DL, Gregory EC. 2016. Cause of fetal death: data from the fetal death report, 2014. Natl Vital Stat Rep 65:1–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

