Overproduction of Chromosomal ampC β-Lactamase Gene Maintains Resistance to Cefazolin in Escherichia coli Isolates
- PMID: 35658712
- PMCID: PMC9241650
- DOI: 10.1128/spectrum.00058-22
Overproduction of Chromosomal ampC β-Lactamase Gene Maintains Resistance to Cefazolin in Escherichia coli Isolates
Abstract
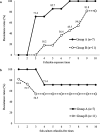
Cefazolin, an active in vitro agent against Escherichia coli, is used to treat urinary and biliary tract infections. Cefazolin is used widely as an antibiotic, and the increase in the emergence of cefazolin-resistant E. coli in many countries is a major concern. We investigated the changes in the susceptibility of E. coli clinical isolates to cefazolin following exposure. A total of 88.9% (16/18 strains) of the strains acquired resistance to cefazolin. All strains with an MIC to cefazolin of 2 μg/mL became resistant. The expression of chromosomal ampC (c-ampC) increased up to 209.1-fold in the resistant strains. Moreover, 11 of the 16 E. coli strains (68.8%) that acquired cefazolin resistance maintained the resistant phenotype after subculture in cefazolin-free medium. Therefore, the acquisition and maintenance of cefazolin resistance in E. coli strains were associated with the overexpression of c-ampC. Mutations in the c-ampC attenuator regions are likely to be maintained and are one of the key factors contributing to the increase in the number of cefazolin-resistant E. coli worldwide. IMPORTANCE This study is the first to demonstrate that mutations in the chromosomal-ampC attenuator region are responsible for the emergence of cefazolin resistance in Escherichia coli strains. The resistance was maintained even after culturing E. coli without cefazolin. This study highlights one of the key factors contributing to the increase in the number of cefazolin-resistant E. coli strains, which can pose a considerable challenge for treating common infections, such as urinary tract infections.
Keywords: Escherichia coli; acquired resistance; cefazolin; chromosomal-ampC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP, Healthcare Infection Control Practices Advisory Committee . 2017. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 152:784–791. doi: 10.1001/jamasurg.2017.0904. - DOI - PubMed
-
- World Health Organization. 2018. Global guidelines for the prevention of surgical site infection. https://www.who.int/publications/i/item/global-guidelines-for-the-preven.... Accessed May 30, 2022. - PubMed
-
- Sharland M, Pulcini C, Harbarth S, Zeng M, Gandra S, Mathur S, Magrini N, 21st WHO Expert Committee on Selection and Use of Essential Medicines . 2018. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect Dis 18:18–20. doi: 10.1016/S1473-3099(17)30724-7. - DOI - PubMed
-
- World Health Organization. 2017. The selection and use of essential medicines. https://apps.who.int/iris/bitstream/handle/10665/259481/9789241210157-en.... Accessed May 30, 2021.
-
- Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM, Society for Healthcare Epidemiology of America . 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177. doi: 10.1086/510393. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

