Risk factors of severe hepatotoxicity among HIV-1 infected individuals initiated on highly active antiretroviral therapy in the Northwest Region of Cameroon
- PMID: 35658835
- PMCID: PMC9166462
- DOI: 10.1186/s12876-022-02305-x
Risk factors of severe hepatotoxicity among HIV-1 infected individuals initiated on highly active antiretroviral therapy in the Northwest Region of Cameroon
Abstract
Background: Hepatotoxicity due to highly active antiretroviral therapy (HAART) has gained prominent attention since it can be affected by many factors. The aim of this study was to determine the prevalence of hepatotoxicity and related risk factors of severe hepatotoxicity following HAART initiation.
Methods: A total of 100 drug-naive patients aged between 18 and 61 years were recruited. They were put on Tenofovir/Lamivudine/Efavirenz [TDF/3TC/EFV] (64), Zidovudine/ Lamivudine/Efavirenz [AZT/3TC/EFV] (22), and Zidovudine/Lamivudine/Nevirapine AZT/3TC/NVP (14) and monitored for 6months and blood samples drawn.Alanine aminotransferases (ALT), aspartate aminotransferases (AST), and alkaline phosphatase (ALP) wereanalyzed by enzymatic methods and used to classify levels of hepatotoxicity.
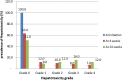
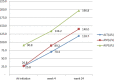
Results: A total of 37(37%) and 49(49%) patients presented with hepatotoxicity while 15% and 28% had severe hepatotoxicity at 4 and 24 weeks respectively. Serum levels of all enzymes increased significantly (p = 0.001) with increased treatment duration. Univariate analysis revealed that the risk factor of developing severe hepatotoxicity was significantly greater in patients < 30years (p = 0.02), males(p = 0.04), low BMI (p = 0.02), low monthly income (p = 0.01) earners, and patients on AZT + 3TC + NVP regimen (p = 0.01). While multivariate analysis at p < 0.09 showed that age 30-40 years, low BMI, low monthly income, and the use of AZT + 3TC + NVP regimen were independent risk factors.
Conclusions: Low BMI, age group of 30-40years, low monthly income, and the use of AZT + 3TC + NVP regimen identified as risk factors for the development of severe hepatotoxicity should be considered as an important strategy by clinicians in preventing the hepatotoxicity.
Keywords: HAART; HIV-1; Hepatotoxicity; Liver enzymes; Risk factors.
© 2022. The Author(s).
Conflict of interest statement
We declare that we have no competing interests.
Figures
References
-
- USAIDS: Global AIDS Update http://www.unaids.org/en/resources/documents. 2016. Assessed on the 13/01/17.
-
- Wambani JR, Ogola PE, Arika WM, Rachuonyo HO, Kemboi NG, Lihana R, et al. Anti-retroviral drug hepatotoxicity and risk factors in HIV patients with or without hepatitis B and C: a review. J Infect Dis Ther. 2015;3:258.
-
- Wenderlein D, Scarcella P. Antiretroviral treatment-associated hepatotoxicity and anemia in patients receiving stavudine or zidovudine containing regimens in Sub- Saharan African Settings. J AIDS Clin Res. 2016;07(01).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical