Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape
- PMID: 35659268
- PMCID: PMC9166526
- DOI: 10.1186/s13045-022-01292-6
Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape
Abstract
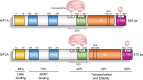
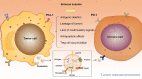
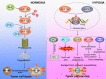
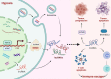
Hypoxia, a common feature of the tumor microenvironment in various types of cancers, weakens cytotoxic T cell function and causes recruitment of regulatory T cells, thereby reducing tumoral immunogenicity. Studies have demonstrated that hypoxia and hypoxia-inducible factors (HIFs) 1 and 2 alpha (HIF1A and HIF2A) are involved in tumor immune escape. Under hypoxia, activation of HIF1A induces a series of signaling events, including through programmed death receptor-1/programmed death ligand-1. Moreover, hypoxia triggers shedding of complex class I chain-associated molecules through nitric oxide signaling impairment to disrupt immune surveillance by natural killer cells. The HIF-1-galactose-3-O-sulfotransferase 1-sulfatide axis enhances tumor immune escape via increased tumor cell-platelet binding. HIF2A upregulates stem cell factor expression to recruit tumor-infiltrating mast cells and increase levels of cytokines interleukin-10 and transforming growth factor-β, resulting in an immunosuppressive tumor microenvironment. Additionally, HIF1A upregulates expression of tumor-associated long noncoding RNAs and suppresses immune cell function, enabling tumor immune escape. Overall, elucidating the underlying mechanisms by which HIFs promote evasion of tumor immune surveillance will allow for targeting HIF in tumor treatment. This review discusses the current knowledge of how hypoxia and HIFs facilitate tumor immune escape, with evidence to date implicating HIF1A as a molecular target in such immune escape. This review provides further insight into the mechanism of tumor immune escape, and strategies for tumor immunotherapy are suggested.
Keywords: Hypoxia; Hypoxia-inducible factors; Immunotherapy; Personalized medicine; Tumor disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

