CEST MRI and MALDI imaging reveal metabolic alterations in the cervical lymph nodes of EAE mice
- PMID: 35659311
- PMCID: PMC9164344
- DOI: 10.1186/s12974-022-02493-z
CEST MRI and MALDI imaging reveal metabolic alterations in the cervical lymph nodes of EAE mice
Abstract
Background: Multiple sclerosis (MS) is a neurodegenerative disease, wherein aberrant immune cells target myelin-ensheathed nerves. Conventional magnetic resonance imaging (MRI) can be performed to monitor damage to the central nervous system that results from previous inflammation; however, these imaging biomarkers are not necessarily indicative of active, progressive stages of the disease. The immune cells responsible for MS are first activated and sensitized to myelin in lymph nodes (LNs). Here, we present a new strategy for monitoring active disease activity in MS, chemical exchange saturation transfer (CEST) MRI of LNs.
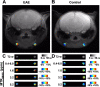
Methods and results: We studied the potential utility of conventional (T2-weighted) and CEST MRI to monitor changes in these LNs during disease progression in an experimental autoimmune encephalomyelitis (EAE) model. We found CEST signal changes corresponded temporally with disease activity. CEST signals at the 3.2 ppm frequency during the active stage of EAE correlated significantly with the cellular (flow cytometry) and metabolic (mass spectrometry imaging) composition of the LNs, as well as immune cell infiltration into brain and spinal cord tissue. Correlating primary metabolites as identified by matrix-assisted laser desorption/ionization (MALDI) imaging included alanine, lactate, leucine, malate, and phenylalanine.
Conclusions: Taken together, we demonstrate the utility of CEST MRI signal changes in superficial cervical LNs as a complementary imaging biomarker for monitoring disease activity in MS. CEST MRI biomarkers corresponded to disease activity, correlated with immune activation (surface markers, antigen-stimulated proliferation), and correlated with LN metabolite levels.
Keywords: CEST MRI; Lymph nodes; Multiple sclerosis; Neuroinflammation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures




References
-
- Kivisäkk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, et al. Human cerebrospinal fluid central memory CD4+T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. - DOI - PMC - PubMed
-
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

