How we treat NK/T-cell lymphomas
- PMID: 35659326
- PMCID: PMC9164389
- DOI: 10.1186/s13045-022-01293-5
How we treat NK/T-cell lymphomas
Abstract
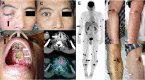
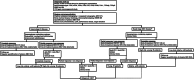
Natural killer (NK)/T-cell lymphomas are aggressive malignancies with a predilection for Asian and South American populations. Epstein-Barr virus (EBV) infection in lymphoma cells is universal. Predominantly extranodal, NK/T-cell lymphomas are divided clinically into nasal (involving the nose and upper aerodigestive tract), non-nasal (involving the skin, gastrointestinal tract, testes, and other organs), and aggressive leukaemia/lymphoma (involving the marrow and multiple organs) subtypes. Initial assessment should include imaging with positron emission tomography computed tomography (PET/CT), quantification of plasma EBV DNA as a surrogate marker of lymphoma load, and bone marrow examination with in situ hybridization for EBV-encoded small RNA. Prognostication can be based on presentation parameters (age, stage, lymph node involvement, clinical subtypes, and EBV DNA), which represent patient factors and lymphoma load; and dynamic parameters during treatment (serial plasma EBV DNA and interim/end-of-treatment PET/CT), which reflect response to therapy. Therapeutic goals are to achieve undetectable plasma EBV DNA and normal PET/CT (Deauville score ≤ 3). NK/T-cell lymphomas express the multidrug resistance phenotype, rendering anthracycline-containing regimens ineffective. Stage I/II nasal cases are treated with non-anthracycline asparaginase-based regimens plus sequential/concurrent radiotherapy. Stage III/IV nasal, and non-nasal and aggressive leukaemia/lymphoma cases are treated with asparaginase-containing regimens and consolidated by allogeneic haematopoietic stem cell transplantation (HSCT) in suitable patients. Autologous HSCT does not improve outcome. In relapsed/refractory cases, novel approaches comprise immune checkpoint blockade of PD1/PD-L1, EBV-specific cytotoxic T-cells, monoclonal antibodies, and histone deacetylase inhibitors. Future strategies may include inhibition of signalling pathways and driver mutations, and immunotherapy targeting the lymphoma and its microenvironment.
Keywords: Aggressive leukaemia/lymphoma; Asparaginase; Immune checkpoint; NK/T-cell lymphoma; Nasal; Non-nasal; PD1; Radiotherapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

