Sorafenib targets and inhibits the oncogenic properties of endometrial cancer stem cells via the RAF/ERK pathway
- PMID: 35659728
- PMCID: PMC9166406
- DOI: 10.1186/s13287-022-02888-y
Sorafenib targets and inhibits the oncogenic properties of endometrial cancer stem cells via the RAF/ERK pathway
Abstract
Background: Distinct subsets of cancer stem cells (CSCs) drive the initiation and progression of malignant tumors via enhanced self-renewal and development of treatment/apoptosis resistance. Endometrial CSC-selective drugs have not been successfully developed because most endometrial cell lines do not contain a sufficient proportion of stable CSCs. Here, we aimed to identify endometrial CSC-containing cell lines and to search for endometrial CSC-selective drugs.
Methods: We first assessed the presence of CSCs by identifying side populations (SPs) in several endometrial cancer cell lines. We then characterized cell viability, colony-formation, transwell invasion and xenotransplantion capability using the isolated SP cells. We also conducted real-time RT-PCR, immunoblot and immunofluorescence analyses of the cells' expression of CSC-associated markers. Focusing on 14 putative CSC-selective drugs, we characterized their effects on the proliferation and apoptosis of endometrial cancer cell lines, examining cell viability and annexin V staining. We further examined the inhibitory effects of the selected drugs, focusing on proliferation, invasion, expression of CSC-associated markers and tumor formation.
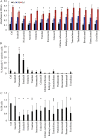
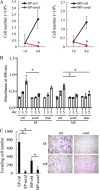
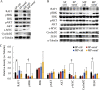
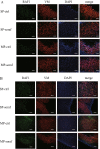
Results: We focused on HHUA cells, an endometrial cancer cell line derived from a well-differentiated endometrial adenocarcinoma. HHUA cells contained a sufficient proportion of stable CSCs with an SP phenotype (HHUA-SP). HHUA-SP showed greater proliferation, colony-formation, and invasive capabilities compared with the main population of HHUA cells (HHUA-MP). HHUA-SP generated larger tumors with higher expression of proliferation-related markers, Ki67, c-MYC and phosphorylated ERK compared with HHUA-MP when transplanted into immunodeficient mice. Among the 14 candidate drugs, sorafenib, an inhibitor of RAF pathways and multiple kinase receptors, inhibited cell proliferation and invasion in both HHUA-SP and -MP, but more profoundly in HHUA-SP. In vivo treatment with sorafenib for 4 weeks reduced the weights of HHUA-SP-derived tumors and decreased the expression of Ki67, ZEB1, and RAF1.
Conclusions: Our results suggest that HHUA is a useful cell line for discovery and identification of endometrial CSC-selective drugs, and that sorafenib may be an effective anti-endometrial cancer drug targeting endometrial CSCs.
Keywords: Cancer stem cells; Endometrial cancer; HHUA; Side-population; Sorafenib.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

