DNA damage to β cells in culture recapitulates features of senescent β cells that accumulate in type 1 diabetes
- PMID: 35660116
- PMCID: PMC9213768
- DOI: 10.1016/j.molmet.2022.101524
DNA damage to β cells in culture recapitulates features of senescent β cells that accumulate in type 1 diabetes
Abstract
Objective: Type 1 Diabetes (T1D) is characterized by progressive loss of insulin-producing pancreatic β cells as a result of autoimmune destruction. In addition to β cell death, recent work has shown that subpopulations of β cells acquire dysfunction during T1D. We previously reported that β cells undergoing a DNA damage response (DDR) and senescence accumulate during the pathogenesis of T1D. However, the question of how senescence develops in β cells has not been investigated.
Methods: Here, we tested the hypothesis that unrepaired DNA damage in the context of genetic susceptibility triggers β cell senescence using culture models including the mouse NIT1 β cell line derived from the T1D-susceptible nonobese diabetic (NOD) strain, human donor islets and EndoC β cells. DNA damage was chemically induced using etoposide or bleomycin and cells or islets were analyzed by a combination of molecular assays for senescence phenotypes including Western blotting, qRT-PCR, Luminex assays, flow cytometry and histochemical staining. RNA-seq was carried out to profile global transcriptomic changes in human islets undergoing DDR and senescence. Insulin ELISAs were used to quantify glucose-stimulated insulin secretion from chemically-induced senescent human islets, EndoC β cells and mouse β cell lines in culture.
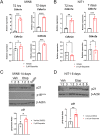
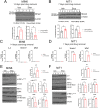
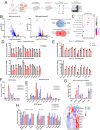
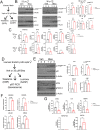
Results: Sub-lethal DNA damage in NIT1 cells led to several classical hallmarks of senescence including sustained DDR activation, growth arrest, enlarged flattened morphology and a senescence-associated secretory phenotype (SASP) resembling what occurs in primary β cells during T1D in NOD mice. These phenotypes differed between NIT1 cells and the MIN6 β cell line derived from a non-T1D susceptible mouse strain. RNA-seq analysis of DNA damage-induced senescence in human islets from two different donors revealed a p53 transcriptional program and upregulation of prosurvival and SASP genes, with inter-donor variability in this response. Inter-donor variability in human islets was also apparent in the extent of persistent DDR activation and SASP at the protein level. Notably, chemically induced DNA damage also led to DDR activation and senescent phenotypes in EndoC-βH5 human β cells, confirming that this response can occur directly in a human β cell line. Finally, DNA damage led to different effects on glucose-stimulated insulin secretion in mouse β cell lines as compared with human islets and EndoC β cells.
Conclusions: Taken together, these findings suggest that some of the phenotypes of senescent β cells that accumulate during the development of T1D in the NOD mouse and humans can be modeled by chemically induced DNA damage to mouse β cell lines, human islets and EndoC β cells in culture. The differences between β cells from different mouse strains and different human islet donors and EndoC β cells highlights species differences and the role for genetic background in modifying the β cell response to DNA damage and its effects on insulin secretion. These culture models will be useful tools to understand some of the mechanisms of β cell senescence in T1D.
Keywords: DNA damage response; Pancreatic β cells; Senescence; Type 1 diabetes.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


Similar articles
-
Alpha cell dysfunction in type 1 diabetes is independent of a senescence program.Front Endocrinol (Lausanne). 2022 Oct 7;13:932516. doi: 10.3389/fendo.2022.932516. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36277717 Free PMC article.
-
Pancreatic beta cell autophagy is impaired in type 1 diabetes.Diabetologia. 2021 Apr;64(4):865-877. doi: 10.1007/s00125-021-05387-6. Epub 2021 Jan 30. Diabetologia. 2021. PMID: 33515072 Free PMC article.
-
Increased inflammation as well as decreased endoplasmic reticulum stress and translation differentiate pancreatic islets from donors with pre-symptomatic stage 1 type 1 diabetes and non-diabetic donors.Diabetologia. 2025 Jul;68(7):1463-1475. doi: 10.1007/s00125-025-06417-3. Epub 2025 Jun 2. Diabetologia. 2025. PMID: 40457096 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Secreted GDF15 maintains transcriptional responses during DNA damage-mediated senescence in human beta cells.Am J Physiol Endocrinol Metab. 2024 Oct 1;327(4):E552-E562. doi: 10.1152/ajpendo.00257.2024. Epub 2024 Aug 28. Am J Physiol Endocrinol Metab. 2024. PMID: 39196800
-
Characterization of the functional and transcriptomic effects of pro-inflammatory cytokines on human EndoC-βH5 beta cells.Front Endocrinol (Lausanne). 2023 Apr 11;14:1128523. doi: 10.3389/fendo.2023.1128523. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37113489 Free PMC article.
-
Autoreactive T Cells and Cytokine Stress Drive β-Cell Senescence Entry and Accumulation in Type 1 Diabetes.Diabetes. 2025 Sep 1;74(9):1562-1576. doi: 10.2337/db24-1122. Diabetes. 2025. PMID: 40498653 Free PMC article.
-
Exploring Transcriptional Regulation of Beta Cell SASP by Brd4-Associated Proteins and Cell Cycle Control Protein p21.Epigenomes. 2024 Mar 6;8(1):10. doi: 10.3390/epigenomes8010010. Epigenomes. 2024. PMID: 38534794 Free PMC article.
-
Body Composition and Senescence: Impact of Polyphenols on Aging-Associated Events.Nutrients. 2024 Oct 25;16(21):3621. doi: 10.3390/nu16213621. Nutrients. 2024. PMID: 39519454 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

