Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer
- PMID: 35660135
- PMCID: PMC9280705
- DOI: 10.1016/j.ccell.2022.05.006
Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer
Abstract
Aerobic exercise is associated with decreased cancer incidence and cancer-associated mortality. However, little is known about the effects of exercise on pancreatic ductal adenocarcinoma (PDA), a disease for which current therapeutic options are limited. Herein, we show that aerobic exercise reduces PDA tumor growth, by modulating systemic and intra-tumoral immunity. Mechanistically, exercise promotes immune mobilization and accumulation of tumor-infiltrating IL15Rα+ CD8 T cells, which are responsible for the tumor-protective effects. In clinical samples, an exercise-dependent increase of intra-tumoral CD8 T cells is also observed. Underscoring the translational potential of the interleukin (IL)-15/IL-15Rα axis, IL-15 super-agonist (NIZ985) treatment attenuates tumor growth, prolongs survival, and enhances sensitivity to chemotherapy. Finally, exercise or NIZ985 both sensitize pancreatic tumors to αPD-1, with improved anti-tumor and survival benefits. Collectively, our findings highlight the therapeutic potential of an exercise-oncology axis and identify IL-15 activation as a promising treatment strategy for this deadly disease.
Trial registration: ClinicalTrials.gov NCT02295956.
Keywords: IL-15; PD-1; T cell; checkpoint blockade; exercise; immunology; immunotherapy; pancreatic cancer; super-agonist.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no competing interests to declare.
Figures


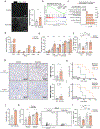
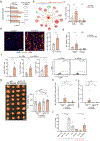

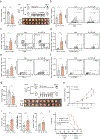


Comment in
-
Antitumor Immunity Is Induced by Exercise in Pancreatic Cancer.Cancer Discov. 2022 Aug 5;12(8):1833. doi: 10.1158/2159-8290.CD-RW2022-107. Cancer Discov. 2022. PMID: 35686838
-
Training the host organism to enhance anti-cancer immunity.Cancer Cell. 2022 Jul 11;40(7):703-705. doi: 10.1016/j.ccell.2022.06.003. Epub 2022 Jun 23. Cancer Cell. 2022. PMID: 35750051
-
Supporting tumor therapy by exercise: boosting T cell immunity by myokines.Signal Transduct Target Ther. 2022 Aug 22;7(1):292. doi: 10.1038/s41392-022-01116-6. Signal Transduct Target Ther. 2022. PMID: 35995771 Free PMC article. No abstract available.
-
A pharmacological strategy to recapitulate exercise-induced antitumoral immunity.Trends Pharmacol Sci. 2022 Dec;43(12):1001-1003. doi: 10.1016/j.tips.2022.08.004. Epub 2022 Aug 30. Trends Pharmacol Sci. 2022. PMID: 36049970
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

