Design and rationale of GUARDD-US: A pragmatic, randomized trial of genetic testing for APOL1 and pharmacogenomic predictors of antihypertensive efficacy in patients with hypertension
- PMID: 35660539
- PMCID: PMC9928488
- DOI: 10.1016/j.cct.2022.106813
Design and rationale of GUARDD-US: A pragmatic, randomized trial of genetic testing for APOL1 and pharmacogenomic predictors of antihypertensive efficacy in patients with hypertension
Abstract
Rationale and objective: APOL1 risk alleles are associated with increased cardiovascular and chronic kidney disease (CKD) risk. It is unknown whether knowledge of APOL1 risk status motivates patients and providers to attain recommended blood pressure (BP) targets to reduce cardiovascular disease.
Study design: Multicenter, pragmatic, randomized controlled clinical trial.
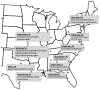
Setting and participants: 6650 individuals with African ancestry and hypertension from 13 health systems.
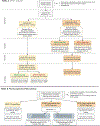
Intervention: APOL1 genotyping with clinical decision support (CDS) results are returned to participants and providers immediately (intervention) or at 6 months (control). A subset of participants are re-randomized to pharmacogenomic testing for relevant antihypertensive medications (pharmacogenomic sub-study). CDS alerts encourage appropriate CKD screening and antihypertensive agent use.
Outcomes: Blood pressure and surveys are assessed at baseline, 3 and 6 months. The primary outcome is change in systolic BP from enrollment to 3 months in individuals with two APOL1 risk alleles. Secondary outcomes include new diagnoses of CKD, systolic blood pressure at 6 months, diastolic BP, and survey results. The pharmacogenomic sub-study will evaluate the relationship of pharmacogenomic genotype and change in systolic BP between baseline and 3 months.
Results: To date, the trial has enrolled 3423 participants.
Conclusions: The effect of patient and provider knowledge of APOL1 genotype on systolic blood pressure has not been well-studied. GUARDD-US addresses whether blood pressure improves when patients and providers have this information. GUARDD-US provides a CDS framework for primary care and specialty clinics to incorporate APOL1 genetic risk and pharmacogenomic prescribing in the electronic health record.
Trial registration: ClinicalTrials.govNCT04191824.
Keywords: Blood pressure; Chronic kidney disease; Genotype; Pharmacogenomics.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest:
The authors have nothing to disclose.
Figures
References
-
- Ostchega YF CD; Nwankwo T; Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. In: SERVICES USDOHAH, ed: National Center for Health Statistics; 2020:1–8. - PubMed
-
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74(10): 1376–1414. - PMC - PubMed
-
- Cheung AK, Chang TI, Cushman WC, et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3): 559–569. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

