Six-month follow-up of a booster dose of CoronaVac in two single-centre phase 2 clinical trials
- PMID: 35660738
- PMCID: PMC9166693
- DOI: 10.1038/s41467-022-30864-w
Six-month follow-up of a booster dose of CoronaVac in two single-centre phase 2 clinical trials
Abstract
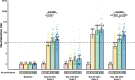
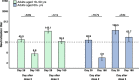
Determining the duration of immunity induced by booster doses of CoronaVac is crucial for informing recommendations for booster regimens and adjusting immunization strategies. In two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials, immunogenicity and safety of four immunization regimens are assessed in adults aged 18 to 59 years and one immunization regimen in adults aged 60 years and older, respectively. Serious adverse events occurring within 6 months after booster doses are recorded as pre-specified secondary endpoints, geometric mean titres (GMTs) of neutralising antibodies one year after the 3-dose schedule immunization and 6 months after the booster doses are assessed as pre-specified exploratory endpoints, GMT fold-decreases in neutralization titres are assessed as post-hoc analyses. Neutralising antibody titres decline approximately 4-fold and 2.5-fold from day 28 to day 180 after third doses in adults aged 18-59 years of age and in adults aged 60 years and older, respectively. No safety concerns are identified during the follow-up period. There are increases in the magnitude and duration of humoral response with homologous booster doses of CoronaVac given 8 months after a primary two-dose immunization series, which could prolong protection and contribute to building our wall of population immunity. Trial number: NCT04352608 and NCT04383574.
© 2022. The Author(s).
Conflict of interest statement
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and Shanghai Roche Pharmaceutical Company. None of those research funding is related to the development of COVID-19 vaccines. Q.X. and T.Y. were the employees of Sinovac Biotech Ltd., L.W. was an employee of Sinovac Life Sciences Co., Ltd. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

