Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma
- PMID: 35660812
- PMCID: PMC10040899
- DOI: 10.1056/NEJMoa2204925
Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma
Abstract
Background: In patients with newly diagnosed multiple myeloma, the effect of adding autologous stem-cell transplantation (ASCT) to triplet therapy (lenalidomide, bortezomib, and dexamethasone [RVD]), followed by lenalidomide maintenance therapy until disease progression, is unknown.
Methods: In this phase 3 trial, adults (18 to 65 years of age) with symptomatic myeloma received one cycle of RVD. We randomly assigned these patients, in a 1:1 ratio, to receive two additional RVD cycles plus stem-cell mobilization, followed by either five additional RVD cycles (the RVD-alone group) or high-dose melphalan plus ASCT followed by two additional RVD cycles (the transplantation group). Both groups received lenalidomide until disease progression, unacceptable side effects, or both. The primary end point was progression-free survival.
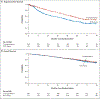
Results: Among 357 patients in the RVD-alone group and 365 in the transplantation group, at a median follow-up of 76.0 months, 328 events of disease progression or death occurred; the risk was 53% higher in the RVD-alone group than in the transplantation group (hazard ratio, 1.53; 95% confidence interval [CI], 1.23 to 1.91; P<0.001); median progression-free survival was 46.2 months and 67.5 months. The percentage of patients with a partial response or better was 95.0% in the RVD-alone group and 97.5% in the transplantation group (P = 0.55); 42.0% and 46.8%, respectively, had a complete response or better (P = 0.99). Treatment-related adverse events of grade 3 or higher occurred in 78.2% and 94.2%, respectively; 5-year survival was 79.2% and 80.7% (hazard ratio for death, 1.10; 95% CI, 0.73 to 1.65).
Conclusions: Among adults with multiple myeloma, RVD plus ASCT was associated with longer progression-free survival than RVD alone. No overall survival benefit was observed. (Funded by the National Heart, Lung, and Blood Institute and others; DETERMINATION ClinicalTrials.gov number, NCT01208662.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
'A stitch in time saves nine': early stem cell transplant continues to improve outcomes in patients with newly diagnosed multiple myeloma.Expert Rev Hematol. 2023 Jul-Dec;16(8):561-563. doi: 10.1080/17474086.2023.2227789. Epub 2023 Jun 23. Expert Rev Hematol. 2023. PMID: 37334674 No abstract available.
References
-
- Kumar SK, Jacobus SJ, Cohen AD, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2020;21:1317–30. - PMC - PubMed
-
- Sonneveld P, Broijl A, Gay F, et al. Bortezomib, lenalidomide, and dexamethasone (VRd) ± daratumumab (DARA) in patients (pts) with transplant-eligible (TE) newly diagnosed multiple myeloma (NDMM): a multicenter, randomized, phase III study (PERSEUS). In: Proceedings and Abstracts of the 2019 ASCO Annual Meeting, May 31–June 4, 2019. Chicago: American Society of Clinical Oncology, 2019. abstract.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
