Dual loss of regulator of G protein signaling 2 and 5 exacerbates ventricular myocyte arrhythmias and disrupts the fine-tuning of Gi/o signaling
- PMID: 35661621
- PMCID: PMC10211374
- DOI: 10.1016/j.yjmcc.2022.05.009
Dual loss of regulator of G protein signaling 2 and 5 exacerbates ventricular myocyte arrhythmias and disrupts the fine-tuning of Gi/o signaling
Abstract
Aims: Cardiac contractility, essential to maintaining proper cardiac output and circulation, is regulated by G protein-coupled receptor (GPCR) signaling. Previously, the absence of regulator of G protein signaling (RGS) 2 and 5, separately, was shown to cause G protein dysregulation, contributing to modest blood pressure elevation and exaggerated cardiac hypertrophic response to pressure-overload. Whether RGS2 and 5 redundantly control G protein signaling to maintain cardiovascular homeostasis is unknown. Here we examined how the dual absence of RGS2 and 5 (Rgs2/5 dbKO) affects blood pressure and cardiac structure and function.
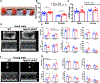
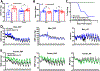
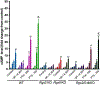
Methods and results: We found that Rgs2/5 dbKO mice showed left ventricular dilatation at baseline by echocardiography. Cardiac contractile response to dobutamine stress test was sex-dependently reduced in male Rgs2/5 dbKO relative to WT mice. When subjected to surgery-induced stress, male Rgs2/5 dbKO mice had 75% mortality within 72-96 h after surgery, accompanied by elevated baseline blood pressure and decreased cardiac contractile function. At the cellular level, cardiomyocytes (CM) from Rgs2/5 dbKO mice showed augmented Ca2+ transients and increased incidence of arrhythmia without augmented contractile response to electrical field stimulation (EFS) and activation of β-adrenergic receptors (βAR) with isoproterenol. Dual loss of Rgs2 and 5 suppressed forskolin-induced cAMP production, which was restored by Gi/o inactivation with pertussis toxin that also reduced arrhythmogenesis during EFS or βAR stimulation. Cardiomyocyte NCX and PMCA mRNA expression was unaffected in Rgs2/5 dbKO male mice. However, there was an exaggerated elevation of EFS-induced cytoplasmic Ca2+ in the presence of SERCA blockade with thapsigargin.
Conclusions: We conclude that RGS2 and 5 promote normal ventricular rhythm by coordinating their regulatory activity towards Gi/o signaling and facilitating cardiomyocyte calcium handling.
Keywords: Arrhythmia; Cardiac excitation-contraction coupling; Cardiomyopathy; Catecholamine; G protein signaling; RGS proteins; cAMP.
Copyright © 2021. Published by Elsevier Ltd.
Figures





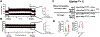
Similar articles
-
Germline deletion of Rgs2 and/or Rgs5 in male mice does not exacerbate left ventricular remodeling induced by subchronic isoproterenol infusion.Physiol Rep. 2025 Jan;13(1):e70178. doi: 10.14814/phy2.70178. Physiol Rep. 2025. PMID: 39746869 Free PMC article.
-
Phosphodiesterases Mediate the Augmentation of Myogenic Constriction by Inhibitory G Protein Signaling and is Negatively Modulated by the Dual Action of RGS2 and 5.Function (Oxf). 2024 Jan 19;5(2):zqae003. doi: 10.1093/function/zqae003. eCollection 2024. Function (Oxf). 2024. PMID: 38486977 Free PMC article.
-
RGS2 is a primary terminator of β₂-adrenergic receptor-mediated G(i) signaling.J Mol Cell Cardiol. 2011 Jun;50(6):1000-7. doi: 10.1016/j.yjmcc.2011.01.015. Epub 2011 Feb 1. J Mol Cell Cardiol. 2011. PMID: 21291891 Free PMC article.
-
Deregulation of RGS2 in cardiovascular diseases.Front Biosci (Schol Ed). 2010 Jan 1;2(2):547-57. doi: 10.2741/s84. Front Biosci (Schol Ed). 2010. PMID: 20036967 Free PMC article. Review.
-
Regulator of G Protein Signaling 2: A Versatile Regulator of Vascular Function.Prog Mol Biol Transl Sci. 2015;133:77-92. doi: 10.1016/bs.pmbts.2015.02.001. Epub 2015 Apr 16. Prog Mol Biol Transl Sci. 2015. PMID: 26123303 Free PMC article. Review.
Cited by
-
Germline deletion of Rgs2 and/or Rgs5 in male mice does not exacerbate left ventricular remodeling induced by subchronic isoproterenol infusion.Physiol Rep. 2025 Jan;13(1):e70178. doi: 10.14814/phy2.70178. Physiol Rep. 2025. PMID: 39746869 Free PMC article.
-
G protein regulation by RGS proteins in the pathophysiology of dilated cardiomyopathy.Am J Physiol Heart Circ Physiol. 2025 Feb 1;328(2):H348-H360. doi: 10.1152/ajpheart.00653.2024. Epub 2025 Jan 7. Am J Physiol Heart Circ Physiol. 2025. PMID: 39772618 Free PMC article. Review.
-
Phosphodiesterases Mediate the Augmentation of Myogenic Constriction by Inhibitory G Protein Signaling and is Negatively Modulated by the Dual Action of RGS2 and 5.Function (Oxf). 2024 Jan 19;5(2):zqae003. doi: 10.1093/function/zqae003. eCollection 2024. Function (Oxf). 2024. PMID: 38486977 Free PMC article.
References
-
- Cadrin-Tourigny J, Shohoudi A, Roy D, Talajic M, Tadros R, Mondesert B, Dyrda K, Rivard L, Andrade JG, Macle L, Guerra PG, Thibault B, Dubuc M, Khairy P, Decreased mortality with beta-blockers in patients with heart failure and coexisting atrial fibrillation: an AF-CHF substudy, JACC Heart Fail. 5 (2017) 99–106. - PubMed
-
- Hepler JR, Gilman AG, proteins G, Trends Biochem. Sci. 17 (1992) 383–387. - PubMed
-
- Chidiac P, Roy AA, Activity, regulation, and intracellular localization of RGS proteins, Recept. Channels 9 (2003) 135–147. - PubMed
-
- Riddle EL, Schwartzman RA, Bond M, Insel PA, Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ. Res 96 (2005) 401–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

