Early human trophoblast development: from morphology to function
- PMID: 35661923
- PMCID: PMC9167809
- DOI: 10.1007/s00018-022-04377-0
Early human trophoblast development: from morphology to function
Abstract
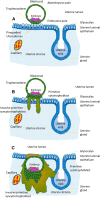
Human pregnancy depends on the proper development of the embryo prior to implantation and the implantation of the embryo into the uterine wall. During the pre-implantation phase, formation of the morula is followed by internalization of blastomeres that differentiate into the pluripotent inner cell mass lineage, while the cells on the surface undergo polarization and differentiate into the trophectoderm of the blastocyst. The trophectoderm mediates apposition and adhesion of the blastocyst to the uterine epithelium. These processes lead to a stable contact between embryonic and maternal tissues, resulting in the formation of a new organ, the placenta. During implantation, the trophectoderm cells start to differentiate and form the basis for multiple specialized trophoblast subpopulations, all of which fulfilling specific key functions in placentation. They either differentiate into polar cells serving typical epithelial functions, or into apolar invasive cells that adapt the uterine wall to progressing pregnancy. The composition of these trophoblast subpopulations is crucial for human placenta development and alterations are suggested to result in placenta-associated pregnancy pathologies. This review article focuses on what is known about very early processes in human reproduction and emphasizes on morphological and functional aspects of early trophoblast differentiation and subpopulations.
Keywords: Blastocyst; Extravillous trophoblast; Syncytialization; Trophectoderm; Villous trophoblast.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests.
Figures


References
-
- Aberkane A, Essahib W, Spits C, De Paepe C, Sermon K, Adriaenssens T, Mackens S, Tournaye H, Brosens JJ, Van de Velde H. Expression of adhesion and extracellular matrix genes in human blastocysts upon attachment in a 2D co-culture system. Mol Hum Reprod. 2018;24:375–387. - PubMed
-
- Allerkamp HH, Clark AR, Lee TC, Morgan TK, Burton GJ, James JL. Something old, something new: digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation. Hum Reprod. 2021;36:571–586. doi: 10.1093/humrep/deaa303. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

