Infiltration of peripheral immune cells into the olfactory bulb in a mouse model of acute nasal inflammation
- PMID: 35661951
- PMCID: PMC9903215
- DOI: 10.1016/j.jneuroim.2022.577897
Infiltration of peripheral immune cells into the olfactory bulb in a mouse model of acute nasal inflammation
Abstract
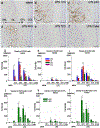
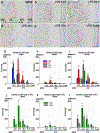
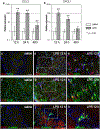
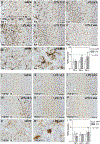
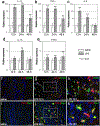
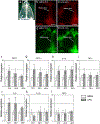
Chronic nasal inflammation induces robust olfactory bulb (OB) atrophy in mice. Here we examined initial events that occur in the OB after bilateral intranasal administration of lipopolysaccharide, focusing on the olfactory nerve fibers and meninges. We analyzed the time course of OB and meninges inflammation using histological and biochemical approaches. Within 12 h, we observed increased chemokine expression and transient infiltration of peripheral immune cells into the OB, resulting in the development of pro-inflammatory status in the OB. Meningeal immunity was activated. Resident microglia produced anti-inflammatory cytokines within 24 h. These could be the initial events that lead to OB atrophy.
Keywords: Meninges; Nasal inflammation; Olfactory bulb; Olfactory nerve fibers; Peripheral immune cells; Resident microglia.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Alves de Lima K, Rustenhoven J, Kipnis J, 2020. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol 38, 597–620. - PubMed
-
- Andonegui G, Zelinski EL, Schubert CL, Knight D, Craig LA, Winston BW, Spanswick SC, Petri B, Jenne CN, Sutherland JC, Nguyen R, Jayawardena N, Kelly MM, Doig CJ, Sutherland RJ, Kubes P, 2018. Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight 3. - PMC - PubMed
-
- Asal N, Bayar Muluk N, Inal M, Sahan MH, Dogan A, Buturak SV, 2018. Olfactory bulbus volume and olfactory sulcus depth in psychotic patients and patients with anxiety disorder/depression. Eur. Arch. Otorhinolaryngol 275, 3017–3024. - PubMed
-
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA, 2016. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U. S. A 113, E1738–E1746. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

