From retina to motoneurons: A substrate for visuomotor transformation in salamanders
- PMID: 35662021
- PMCID: PMC9545292
- DOI: 10.1002/cne.25348
From retina to motoneurons: A substrate for visuomotor transformation in salamanders
Abstract
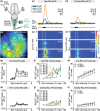
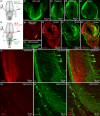
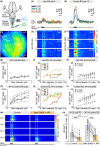
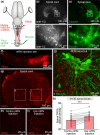
The transformation of visual input into motor output is essential to approach a target or avoid a predator. In salamanders, visually guided orientation behaviors have been extensively studied during prey capture. However, the neural circuitry involved is not resolved. Using salamander brain preparations, calcium imaging and tracing experiments, we describe a neural substrate through which retinal input is transformed into spinal motor output. We found that retina stimulation evoked responses in reticulospinal neurons of the middle reticular nucleus, known to control steering movements in salamanders. Microinjection of glutamatergic antagonists in the optic tectum (superior colliculus in mammals) decreased the reticulospinal responses. Using tracing, we found that retina projected to the dorsal layers of the contralateral tectum, where the dendrites of neurons projecting to the middle reticular nucleus were located. In slices, stimulation of the tectal dorsal layers evoked glutamatergic responses in deep tectal neurons retrogradely labeled from the middle reticular nucleus. We then examined how tectum activation translated into spinal motor output. Tectum stimulation evoked motoneuronal responses, which were decreased by microinjections of glutamatergic antagonists in the contralateral middle reticular nucleus. Reticulospinal fibers anterogradely labeled from tracer injection in the middle reticular nucleus were preferentially distributed in proximity with the dendrites of ipsilateral motoneurons. Our work establishes a neural substrate linking visual and motor centers in salamanders. This retino-tecto-reticulo-spinal circuitry is well positioned to control orienting behaviors. Our study bridges the gap between the behavioral studies and the neural mechanisms involved in the transformation of visual input into motor output in salamanders.
Keywords: motoneurons; reticulospinal neurons; retina; salamander; spinal cord; tectum; visuomotor.
© 2022 The Authors. The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures


Similar articles
-
Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum.J Neurosci. 2005 Oct 5;25(40):9294-303. doi: 10.1523/JNEUROSCI.2678-05.2005. J Neurosci. 2005. PMID: 16207889 Free PMC article.
-
Tectal activation of premotor and motor networks during feeding in salamanders.Eur J Morphol. 1994 Aug;32(2-4):106-16. Eur J Morphol. 1994. PMID: 7803155
-
Connectivity of the goldfish optic tectum with the mesencephalic and rhombencephalic reticular formation.Exp Brain Res. 2003 Jul;151(1):123-35. doi: 10.1007/s00221-003-1432-6. Epub 2003 May 14. Exp Brain Res. 2003. PMID: 12748838
-
How visual inputs to the ponto-bulbar reticular formation are used in the synthesis of premotor signals during orienting.Prog Brain Res. 1989;80:159-70; discussion 127-8. doi: 10.1016/s0079-6123(08)62209-8. Prog Brain Res. 1989. PMID: 2699363 Review.
-
Roles of periventricular neurons in retinotectal transmission in the optic tectum.Prog Neurobiol. 2006 Jun;79(2):112-21. doi: 10.1016/j.pneurobio.2006.06.002. Epub 2006 Aug 9. Prog Neurobiol. 2006. PMID: 16901616 Review.
Cited by
-
Adeno-associated viral tools to trace neural development and connectivity across amphibians.Dev Cell. 2025 Mar 10;60(5):794-812.e6. doi: 10.1016/j.devcel.2024.10.025. Epub 2024 Nov 26. Dev Cell. 2025. PMID: 39603234
-
Cell-type specific sensory and motor activity in the cuneiform nucleus and pedunculopontine nucleus in mice.Sci Rep. 2025 Jul 1;15(1):20408. doi: 10.1038/s41598-025-05572-2. Sci Rep. 2025. PMID: 40594670 Free PMC article.
References
-
- Bachmann, L. C. , Matis, A. , Lindau, N. T. , Felder, P. , Gullo, M. , & Schwab, M. E. (2013). Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Science Translational Medicine, 5, 208ra146. - PubMed
-
- Beauséjour, P.‐A. , & Zielinski, B. , Dubuc, R. (2021). Olfactory‐induced locomotion in lampreys. Cell and Tissue Research. - PubMed
Publication types
MeSH terms
Grants and funding
- RGPIN-2017-05522/Natural Sciences and Engineering Research Council of Canada
- RTI-2019-00628/Natural Sciences and Engineering Research Council of Canada
- 407083/The Canadian Institutes of Health Research
- 34920/The Fonds de la Recherche du Québec - Santé
- 36772/The Fonds de la Recherche du Québec - Santé
- 297299/Doctoral fellowship
- 39344/The Canada Foundation for Innovation
- The Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke
- The Faculté de médecine et des sciences de la santé
- The Centre d'excellence en Neurosciences de l'Université de Sherbooke
- The fonds Jean-Luc Mongrain de la fondation du CHUS
- 951477/The European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme
LinkOut - more resources
Full Text Sources
Miscellaneous

