Artificial intelligence for assessment of Stargardt macular atrophy
- PMID: 35662193
- PMCID: PMC9165405
- DOI: 10.4103/1673-5374.339477
Artificial intelligence for assessment of Stargardt macular atrophy
Abstract
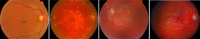
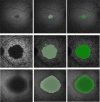
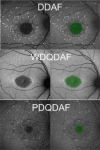
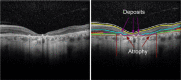
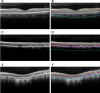
Stargardt disease (also known as juvenile macular degeneration or Stargardt macular degeneration) is an inherited disorder of the retina, which can occur in the eyes of children and young adults. It is the most prevalent form of juvenile-onset macular dystrophy, causing progressive (and often severe) vision loss. Images with Stargardt disease are characterized by the appearance of flecks in early and intermediate stages, and the appearance of atrophy, due to cells wasting away and dying, in the advanced stage. The primary measure of late-stage Stargardt disease is the appearance of atrophy. Fundus autofluorescence is a widely available two-dimensional imaging technique, which can aid in the diagnosis of the disease. Spectral-domain optical coherence tomography, in contrast, provides three-dimensional visualization of the retinal microstructure, thereby allowing the status of the individual retinal layers. Stargardt disease may cause various levels of disruption to the photoreceptor segments as well as other outer retinal layers. In recent years, there has been an exponential growth in the number of applications utilizing artificial intelligence for help with processing such diseases, heavily fueled by the amazing successes in image recognition using deep learning. This review regarding artificial intelligence deep learning approaches for the Stargardt atrophy screening and segmentation on fundus autofluorescence images is first provided, followed by a review of the automated retinal layer segmentation with atrophic-appearing lesions and fleck features using artificial intelligence deep learning construct. The paper concludes with a perspective about using artificial intelligence to potentially find early risk factors or biomarkers that can aid in the prediction of Stargardt disease progression.
Keywords: Stargardt atrophy; Stargardt disease; Stargardt flecks; artificial intelligence; assessment; deep learning; fundus autofluorescence; screening; segmentation; spectral-domain optical coherence tomography.
Conflict of interest statement
Figures

References
-
- Binley K, Widdowson P, Loader J, Kelleher M, Iqball S, Ferrige G, de Belin J, Carlucci M, Angell-Manning D, Hurst F, Ellis S, Miskin J, Fernandes A, Wong P, Allikmets R, Bergstrom C, Aaberg T, Yan J, Kong J, Gouras P, et al. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors:safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci. 2013;54:4061–4071. - PMC - PubMed
-
- Cabrera Fernandez D, Salinas HM, Puliafito CA. Automated detection of retinal layer structures on optical coherence tomography images. Opt Express. 2005;13:10200–10216. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

