Necrostatin-1 decreases necroptosis and inflammatory markers after intraventricular hemorrhage in mice
- PMID: 35662218
- PMCID: PMC9165399
- DOI: 10.4103/1673-5374.339488
Necrostatin-1 decreases necroptosis and inflammatory markers after intraventricular hemorrhage in mice
Abstract
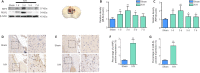
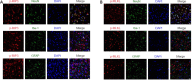
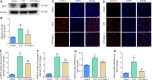
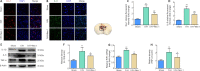
Necrostatin-1, an inhibitor of necroptosis, can effectively inhibit necrotic apoptosis in neurological diseases, which results in the inhibition of inflammation, endoplasmic reticulum stress, and reactive oxygen species production and substantial improvement of neurological function. However, the effects of necrostatin-1 on intraventricular hemorrhage (IVH) remain unknown. In this study, we established a mouse model of IVH by injecting autologous blood into the lateral ventricle of the brain. We also injected necrostatin-1 into the lateral ventricle one hour prior to IVH induction. We found that necrostatin-1 effectively reduced the expression levels of the necroptosis markers receptor-interacting protein kinase (RIP)1, RIP3, mixed lineage kinase domain-like protein (MLKL), phosphorylated (p)-RIP3, and p-MLKL and the levels of interleukin-1β , interleukin-6, and tumor necrosis factor-α in the surrounding areas of the lateral ventricle. However, necrostatin-1 did not reduce ependymal ciliary injury or brain water content. These findings suggest that necrostatin-1 can prevent local inflammation and microglial activation induced by IVH but does not greatly improve prognosis.
Keywords: MLKL; RIP1; RIP3; ependymal cilia; hydrocephalus; inflammation; intraventricular hemorrhage; microglia; necroptosis; necrostatin-1.
Conflict of interest statement
None
Figures

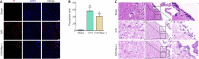

References
-
- Berger SB, Harris P, Nagilla R, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Ouellette M, King BW, Wisnoski D, Cox J, Reilly M, Marquis RW, Bertin J, Gough PJ. Characterization of GSK'963:a structurally distinct, potent and selective inhibitor of RIP1 kinase. Cell Death Discov. 2015;1:15009. - PMC - PubMed
-
- Bosche B, Mergenthaler P, Doeppner TR, Hescheler J, Molcanyi M. Complex clearance mechanisms after intraventricular hemorrhage and rt-PA treatment-a review on clinical trials. Transl Stroke Res. 2020;11:337–344. - PubMed
-
- Caccamo A, Branca C, Piras IS, Ferreira E, Huentelman MJ, Liang WS, Readhead B, Dudley JT, Spangenberg EE, Green KN, Belfiore R, Winslow W, Oddo S. Necroptosis activation in Alzheimer's disease. Nat Neurosci. 2017;20:1236–1246. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

