NR4A1 agonist cytosporone B attenuates neuroinflammation in a mouse model of multiple sclerosis
- PMID: 35662227
- PMCID: PMC9165396
- DOI: 10.4103/1673-5374.339492
NR4A1 agonist cytosporone B attenuates neuroinflammation in a mouse model of multiple sclerosis
Abstract
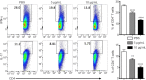
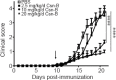
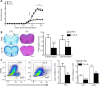
Nuclear receptor subfamily 4 group A1 (NR4A1) is an orphan nuclear receptor, which is expressed in the majority of cells. NR4A1 expression in peripheral blood mononuclear cells is low during the preclinical stage of multiple sclerosis. Knockout of the Nr4a1 gene in mice can aggravate the symptoms of experimental autoimmune encephalomyelitis (EAE), which is an animal model of multiple sclerosis. In this study, we intragastrically administered the NR4A1 agonist cytosporone B (Csn-B) to mice after inducing EAE. After treatment with Csn-B, the clinical symptoms in the EAE mice were substantially attenuated compared with that in PBS-treated control mice. The percentages of CD4+ T cells and F4/80+ cells in the central nervous system were decreased. In addition, interferon-γ and interleukin-17 production by proinflammatory Th1/Th17 cells in the central nervous system and interferon-γ levels in splenocytes were decreased after Csn-B treatment. These findings suggest that the NR4A1 agonist Csn-B can alleviate nerve injury after EAE induction, and, therefore, may be useful as a potential treatment for multiple sclerosis.
Keywords: NR4A1; NR4A1 agonist; Th1; Th17; cytosporone B (Csn-B); experimental autoimmune encephalomyelitis; macrophages; microglia; multiple sclerosis; treatment.
Conflict of interest statement
None
Figures



Similar articles
-
Nr4a1 plays a crucial modulatory role in Th1/Th17 cell responses and CNS autoimmunity.Brain Behav Immun. 2018 Feb;68:44-55. doi: 10.1016/j.bbi.2017.09.015. Epub 2017 Sep 27. Brain Behav Immun. 2018. PMID: 28962999
-
Cytosporone B (Csn-B), an NR4A1 agonist, attenuates acute cardiac allograft rejection by inducing differential apoptosis of CD4+T cells.Int Immunopharmacol. 2022 Mar;104:108521. doi: 10.1016/j.intimp.2022.108521. Epub 2022 Jan 10. Int Immunopharmacol. 2022. PMID: 35026656
-
Treatment with the NR4A1 agonist cytosporone B controls influenza virus infection and improves pulmonary function in infected mice.PLoS One. 2017 Oct 20;12(10):e0186639. doi: 10.1371/journal.pone.0186639. eCollection 2017. PLoS One. 2017. PMID: 29053748 Free PMC article.
-
Cytosporone B is an agonist for nuclear orphan receptor Nur77.Nat Chem Biol. 2008 Sep;4(9):548-56. doi: 10.1038/nchembio.106. Nat Chem Biol. 2008. PMID: 18690216
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
Cited by
-
Characterization of the Immune Microenvironment and Identification of Biomarkers in Chronic Rhinosinusitis with Nasal Polyps Using Single-Cell RNA Sequencing and Transcriptome Analysis.J Inflamm Res. 2024 Jan 12;17:253-277. doi: 10.2147/JIR.S440409. eCollection 2024. J Inflamm Res. 2024. PMID: 38229690 Free PMC article.
-
Controversy and multiple roles of the solitary nucleus receptor Nur77 in disease and physiology.FASEB J. 2025 Mar 31;39(6):e70468. doi: 10.1096/fj.202402775RR. FASEB J. 2025. PMID: 40079203 Free PMC article. Review.
-
Transcriptomic analysis of rat prefrontal cortex following chronic stress induced by social isolation - Relevance to psychiatric and neurodevelopmental illness, and implications for treatment.Neurobiol Stress. 2024 Oct 17;33:100679. doi: 10.1016/j.ynstr.2024.100679. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39502833 Free PMC article.
-
Impact of the Voltage-Gated Calcium Channel Antagonist Nimodipine on the Development of Oligodendrocyte Precursor Cells.Int J Mol Sci. 2023 Feb 13;24(4):3716. doi: 10.3390/ijms24043716. Int J Mol Sci. 2023. PMID: 36835129 Free PMC article.
-
Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis.Neural Regen Res. 2024 Mar;19(3):671-679. doi: 10.4103/1673-5374.379050. Neural Regen Res. 2024. PMID: 37721300 Free PMC article.
References
-
- Achiron A, Grotto I, Balicer R, Magalashvili D, Feldman A, Gurevich M. Microarray analysis identifies altered regulation of nuclear receptor family members in the pre-disease state of multiple sclerosis. Neurobiol Dis. 2010;38:201–209. - PubMed
-
- Balasa R, Barcutean L, Balasa A, Motataianu A, Roman-Filip C, Manu D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum Immunol. 2020;81:237–243. - PubMed
-
- Benarroch EE. Microglia:Multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81:1079–1088. - PubMed
-
- Bogie JF, Stinissen P, Hendriks JJ. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 2014;128:191–213. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

