Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial
- PMID: 35662283
- PMCID: PMC9205784
- DOI: 10.1038/s41591-022-01829-9
Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial
Abstract
Chemotherapy combined with immunotherapy has improved the treatment of certain solid tumors, but effective regimens remain elusive for pancreatic ductal adenocarcinoma (PDAC). We conducted a randomized phase 2 trial evaluating the efficacy of nivolumab (nivo; anti-PD-1) and/or sotigalimab (sotiga; CD40 agonistic antibody) with gemcitabine/nab-paclitaxel (chemotherapy) in patients with first-line metastatic PDAC ( NCT03214250 ). In 105 patients analyzed for efficacy, the primary endpoint of 1-year overall survival (OS) was met for nivo/chemo (57.7%, P = 0.006 compared to historical 1-year OS of 35%, n = 34) but was not met for sotiga/chemo (48.1%, P = 0.062, n = 36) or sotiga/nivo/chemo (41.3%, P = 0.223, n = 35). Secondary endpoints were progression-free survival, objective response rate, disease control rate, duration of response and safety. Treatment-related adverse event rates were similar across arms. Multi-omic circulating and tumor biomarker analyses identified distinct immune signatures associated with survival for nivo/chemo and sotiga/chemo. Survival after nivo/chemo correlated with a less suppressive tumor microenvironment and higher numbers of activated, antigen-experienced circulating T cells at baseline. Survival after sotiga/chemo correlated with greater intratumoral CD4 T cell infiltration and circulating differentiated CD4 T cells and antigen-presenting cells. A patient subset benefitting from sotiga/nivo/chemo was not identified. Collectively, these analyses suggest potential treatment-specific correlates of efficacy and may enable biomarker-selected patient populations in subsequent PDAC chemoimmunotherapy trials.
© 2022. The Author(s).
Conflict of interest statement
A.H.K., E.M.O., R.H.V., M.H.O., G.F. and E.J.W. report grants from the Parker Institute for Cancer Immunotherapy (PICI) during the conduct of this study. A.H.K. reports grants from Celgene, Apexigen and Bristol Myers Squibb (BMS) outside the submitted work. E.M.O. reports research funding from MSK, Genentech/Roche, Celgene/BMS, BioNTech, AstraZeneca, Arcus and Elicio and consulting/DSMB for Cytomx Therapeutics, Rafael Therapeutics, Silenseed, Tyme, Seagen, Boehringer Ingelheim, BioNTech, Ipsen, Merck, IDEAYA, AstraZeneca, Noxxon, BioSapien, Cend Therapeutics, Thetis, Bayer (spouse), Genentech/Roche (spouse), Celgene/BMS (spouse) and Eisai (spouse). L.H.B. declares the following unrelated advisory activities: StemImmune/Calidi, Western Oncolytics, Torque Therapeutics, Khloris, Pyxis, Cytomix, DCprime, RAPT, Takeda and EnaraBio. O.R. reports personal fees from Merck, Celgene, Five Prime Therapeutics, GlaxoSmithKline, Bayer, Roche/Genentech, Puretech, Imvax and Sobi outside the submitted work and has a patent pending for methods that make use of pembrolizumab and trebananib. P.F.G. reports stock ownership in Teiko.bio. R.H.V. reports grants from FibroGen, Inovio, Janssen and Eli Lilly and personal fees from MedImmune, Eli Lilly, Celgene, Celldex Therapeutics and Verastem Oncology outside the submitted work; is an inventor on a licensed patent relating to cancer cellular immunotherapy and cancer vaccines; and receives royalties from Children’s Hospital Boston for a licensed research-only monoclonal antibody. R.O.C. is an employee of Personalis, a company that PICI paid to produce sequence information for some samples reported in this paper as part of a collaboration. R.O.C. is also an inventor on US patent number 09183496 issued to Personalis, which describes the genomic analyses in the Personalis sequencing platform used to sequence the samples in this study. T.M.L. reports Coherus Biosciences employment; LISCure Biosciences Scientific Advisory Board membership; stock ownership in AstraZenca; and consulting outside the submitted work for Grey Wolf Therapeutics and BiOneCure. V.M.H.-L. is an employee of BMS and holds stock. Z.A.W. reports grants from Novartis, Five Prime Therapeutics, Plexxikon and BMS and personal fees from Merck, Eli Lilly, Daiichi, AstraZeneca and Bayer outside the submitted work. M.S. reports consulting for Natera. M.H.O. reports grants from BMS and Celldex; grants and non-financial support from Stand Up To Cancer; and personal fees from Natera outside the submitted work. M.T. is an employee of Biognosys AG. G.F. reports personal fees from Merck, Roche/Genentech and CytomX outside the submitted work; and his spouse owns stock in Seattle Genetics. E.J.W. is a consultant or an advisor for Merck, Elstar, Janssen, Related Sciences, Synthekine and Surface Oncology; is a founder of Surface Oncology and Arsenal Biosciences; and is an inventor on US patent number 10,370,446, submitted by Emory University that covers the use of PD-1 blockade to treat infections and cancer. L.J.P., D.M.M., R.A.W., J.P.L., C.R.C., J.X.Y., S.M.P., J.X., J.K., R.M., C.A., K.T.B., T.J.H., J.S.M., D.D.J., X.Y., L.S., U.D., J.O.-T., R.I., J.F. and S.B. report no competing interests related to the work presented.
Figures

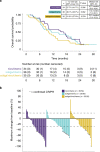




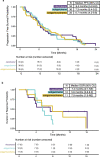






Comment in
-
Two types of biomarker-dependent chemo-immunotherapy for pancreatic cancer?Cell Rep Med. 2022 Oct 18;3(10):100788. doi: 10.1016/j.xcrm.2022.100788. Cell Rep Med. 2022. PMID: 36260984 Free PMC article.
References
-
- Rahib L, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Sharma P, et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11:838–857. - PubMed
-
- Patnaik A, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin. Cancer Res. 2015;21:4286–4293. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

