Hyaluronan synthase 2, a target of miR-200c, promotes carbon tetrachloride-induced acute and chronic liver inflammation via regulation of CCL3 and CCL4
- PMID: 35662287
- PMCID: PMC9256637
- DOI: 10.1038/s12276-022-00781-5
Hyaluronan synthase 2, a target of miR-200c, promotes carbon tetrachloride-induced acute and chronic liver inflammation via regulation of CCL3 and CCL4
Abstract
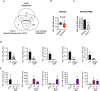
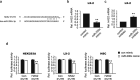
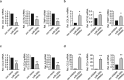
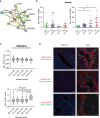
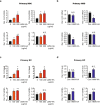
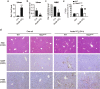
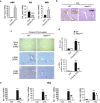
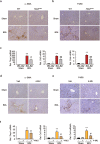
Liver fibrosis occurs during wound healing after repeated liver injury and is characterized by extensive extracellular matrix deposition. We previously identified hyaluronan synthase 2 (HAS2) as a driver of liver fibrosis and hepatic stellate cell (HSC) activation. Developing strategies to suppress HSC activation is key to alleviating liver fibrosis, and HAS2 is an attractive candidate for intervention. To gain insight into the molecular function of HAS2, we investigated its posttranscriptional regulation. We found that miR-200c directly targets the 3' untranslated regions of HAS2. Moreover, miR-200c and HAS2 were inversely expressed in fibrotic human and mouse livers. After establishing the direct interaction between miR-200c and HAS2, we investigated the functional outcome of regulating HAS2 expression in three murine models: CCl4-induced acute liver injury, CCl4-induced chronic liver fibrosis, and bile duct ligation-induced liver fibrosis. Hepatic Has2 expression was induced by acute and chronic CCl4 treatment. In contrast, miR-200c expression was decreased after CCl4 treatment. HSC-specific Has2 deletion reduced the expression of inflammatory markers and infiltration of macrophages in the models. Importantly, hyaluronidase-2 (HYAL2) but not HYAL1 was overexpressed in fibrotic human and murine livers. HYAL2 is an enzyme that can cleave the extracellular matrix component hyaluronan. We found that low-molecular-weight hyaluronan stimulated the expression of inflammatory genes. Treatment with the HA synthesis inhibitor 4-methylumbelliferone alleviated bile duct ligation-induced expression of these inflammatory markers. Collectively, our results suggest that HAS2 is negatively regulated by miR-200c and contributes to the development of acute liver injury and chronic liver inflammation via hyaluronan-mediated immune signaling.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Collaborators, G. B. D. C. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

