Recent insights into SARS-CoV-2 omicron variant
- PMID: 35662313
- PMCID: PMC9347414
- DOI: 10.1002/rmv.2373
Recent insights into SARS-CoV-2 omicron variant
Abstract
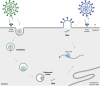
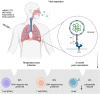
The SARS-CoV-2 omicron variant (B.1.1.529) was first identified in Botswana and South Africa, and its emergence has been associated with a steep increase in the number of SARS-CoV-2 infections. The omicron variant has subsequently spread very rapidly across the world, resulting in the World Health Organization classification as a variant of concern on 26 November 2021. Since its emergence, great efforts have been made by research groups around the world that have rapidly responded to fill our gaps in knowledge for this novel variant. A growing body of data has demonstrated that the omicron variant shows high transmissibility, robust binding to human angiotensin-converting enzyme 2 receptor, attenuated viral replication, and causes less severe disease in COVID-19 patients. Further, the variant has high environmental stability, high resistance against most therapeutic antibodies, and partial escape neutralisation by antibodies from convalescent patients or vaccinated individuals. With the pandemic ongoing, there is a need for the distillation of literature from primary research into an accessible format for the community. In this review, we summarise the key discoveries related to the SARS-CoV-2 omicron variant, highlighting the gaps in knowledge that guide the field's ongoing and future work.
Keywords: COVID-19; SARS-CoV-2; coronavirus; omicron; pandemic; variants of concern.
© 2022 The Authors. Reviews in Medical Virology published by John Wiley & Sons Ltd.
Conflict of interest statement
No conflict of interest declared.
Figures

References
-
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. ISSN 1476‐4687. https://www.ncbi.nlm.nih.gov/pubmed/32015507 - PMC - PubMed
-
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. ISSN 1533‐4406. https://www.ncbi.nlm.nih.gov/pubmed/31978945 - PMC - PubMed
-
- Zhong NS, Zheng B, Li Y, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362(9393):1353‐1358. ISSN 1474‐547X. https://www.ncbi.nlm.nih.gov/pubmed/14585636 - PMC - PubMed
-
- Zaki AM, vanBoheemen S, Bestebroer TM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. ISSN 1533‐4406. https://www.ncbi.nlm.nih.gov/pubmed/23075143 - PubMed
-
- WHO. Coronavirus Disease 2019 (COVID‐19); 2020. Situation Report ‐ 51.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

