Process mapping strategies to prevent subcutaneous implantable cardioverter-defibrillator infections
- PMID: 35662315
- PMCID: PMC9544305
- DOI: 10.1111/jce.15566
Process mapping strategies to prevent subcutaneous implantable cardioverter-defibrillator infections
Abstract
Background: Infection remains a major complication of cardiac implantable electronic devices and can lead to significant morbidity and mortality. Implantable devices that avoid transvenous leads, such as the subcutaneous implantable cardioverter-defibrillator (S-ICD), can reduce the risk of serious infection-related complications, such as bloodstream infection and infective endocarditis. While the 2017 AHA/ACC/HRS guidelines include recommendations for S-ICD use for patients at high risk of infection, currently, there are no clinical trial data that address best practices for the prevention of S-ICD infections. Therefore, an expert panel was convened to develop a consensus on these topics.
Methods: An expert process mapping methodology was used to achieve consensus on the appropriate steps to minimize or prevent S-ICD infections. Two face-to-face meetings of high-volume S-ICD implanters and an infectious diseases specialist, with expertise in cardiovascular implantable electronic device infections, were conducted to develop consensus on useful strategies pre-, peri-, and postimplant to reduce S-ICD infection risk.
Results: Expert panel consensus on recommended steps for patient preparation, S-ICD implantation, and postoperative management was developed to provide guidance in individual patient management.
Conclusion: Achieving expert panel consensus by process mapping methodology for S-ICD infection prevention was attainable, and the results should be helpful to clinicians in adopting interventions to minimize risks of S-ICD infection.
Keywords: antibiotic prophylaxis; antibiotics; defibrillator; infection; mapping; prevention; subcutaneous implantable cardioverter-defibrillator; surgical site infection.
© 2022 The Authors. Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Figures


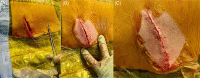
References
-
- Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter‐defibrillator. N Engl J Med. 2010;363(1):36‐44. - PubMed
-
- Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable‐cardioverter defibrillator. Circulation. 2013;128(9):944‐953. - PubMed
-
- Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2‐year results from a pooled analysis of the IDE study and effortless registry. J Am Coll Cardiol. 2015;65(16):1605‐1615. - PubMed
-
- Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383(6):526‐536. - PubMed
-
- Al‐Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2017;15(10):e190‐e252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

