Risks related to a possible reduction of the waiting period for dogs after rabies antibody titration to 30 days compared with 90 days of the current EU legislative regime
- PMID: 35662806
- PMCID: PMC9161159
- DOI: 10.2903/j.efsa.2022.7350
Risks related to a possible reduction of the waiting period for dogs after rabies antibody titration to 30 days compared with 90 days of the current EU legislative regime
Abstract
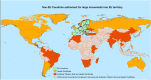
EFSA received a mandate from the European Commission to assess the risks related to a possible reduction of the waiting period after rabies antibody titration test to 30 days compared with 90 days of the current EU legislation, for dogs moving from certain non-EU countries to the EU. This Scientific Report assessed the probability of introduction of rabies into the EU through commercial and non-commercial movements of vaccinated dogs with a positive titration test (≥ 0.5 IU/mL) if the waiting period decreases from 90 to 30 days. Assuming that all the legal requirements are complied with, the risk of transmission of rabies through the movement of a vaccinated dog is related to the risk of introducing an animal incubating rabies that was infected before the day of vaccination or shortly after vaccination but before the development of immunity (21 days post-vaccination). Using published data on the incubation period for experimental and field cases in dogs and considering the rabies incidence data in certain countries, the aggregated probability for the annual introduction of rabies through dogs was assessed. Considering the uncertainty related to the duration of the incubation period, the number of imported dogs, and the disease incidence in some countries it was concluded with a 95% certainty that the maximum number of rabies-infected imported dogs complying with the regulations in a 20-year period could increase from 5 to 20 when decreasing the waiting period from 90 to 30 days. Nevertheless, the potential impact of even a small increase in probability means the risk is increased for a region like the EU where rabies has long been a focus for eradication, to protect human and animal health.
Keywords: antibody titration test; dog; import; rabies; vaccination; waiting period.
© 2022 Wiley‐VCH Verlag GmbH & Co. KgaA on behalf of the European Food Safety Authority.
Figures



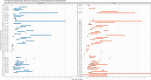
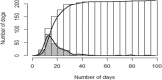
Each group of dogs has a unique identification code and was inoculated with different doses of RABV through intramuscular (IM), intracerebral (IC) and intranasal (IN) route. Black dots indicate the time to the onset of clinical signs and death individually for each dog of the group. For the group with code number W/1, the last dog died 257 days post‐inoculation but to maintain better visualisation of the whole graph the x‐axis is not extended up to the value 257.


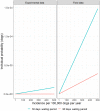
The Incidence Rate (IR) is calculated as the number of infected dogs per 100,000 dogs per year. When the incubation period is derived from the experimental data and the waiting period is 90 days, the individual probability is zero regardless the value of the IR.

Incidence Rate (IR) is calculated as the number of infected dogs per 100,000 dogs per year. When the incubation period is derived from the experimental data for a waiting period of 90 days, the individual probability is zero regardless the value of the IR.
References
-
- Arega S, Conan A, Sabeta CT, Crafford JE, Wentzel J, Reininghaus B, Biggs L, Leisewitz AL, Quan M, Toka F and Knobel DL, 2020. Rabies vaccination of 6‐week‐old puppies born to immunized mothers: a randomized controlled trial in a high‐mortality population of owned, free‐roaming dogs. Trop Med Infect Dis, 5. 10.3390/tropicalmed5010045 - DOI - PMC - PubMed
-
- Asokkumar M, Ramesh A, Gunaseelan L, Tirumurugaan KG and Anuradha P, 2014. Comparison of two different methods to quantify the neutralizing rabies antibodies in vaccinated dogs. Indian Veterinary Journal, 91, 23–25.
-
- Asokkumar M, Ganesan PI, Sekar M, Anuradha P and Balakrishnan S, 2016. Vaccination studies against rabies in farm and pet animals using different immunization routes. Indian Veterinary Journal, 93, 33–36.
-
- Bahloul C, Taieb D, Diouani MF, Ahmed SBH, Chtourou Y, B'chir BI, Kharmachi H and Dellagi K, 2006. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine, 24, 1063–1072. 10.1016/j.vaccine.2005.09.016 - DOI - PubMed
LinkOut - more resources
Full Text Sources
