Osteocyte Apoptosis, Bone Marrow Adiposity, and Fibrosis in the Irradiated Human Mandible
- PMID: 35662809
- PMCID: PMC9156996
- DOI: 10.1016/j.adro.2022.100951
Osteocyte Apoptosis, Bone Marrow Adiposity, and Fibrosis in the Irradiated Human Mandible
Abstract
Purpose: To assess the effect of radiation therapy on osteocyte apoptosis, osteocyte death, and bone marrow adipocytes in the human mandible and its contribution to the pathophysiology of radiation damage to the mandibular bone.
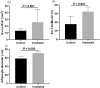
Methods and materials: Mandibular cancellous bone biopsies were taken from irradiated patients and nonirradiated controls. Immunohistochemical detection of cleaved caspase-3 was performed to visualize apoptotic osteocytes. The number of apoptotic osteocytes per bone area and per total amount of osteocytes, osteocytes per bone area, and empty lacunae per bone area were counted manually. The percentage fibrotic tissue and adipose tissue per bone marrow area, the percentage bone marrow of total area, and the mean adipocyte diameter (μm) was determined digitally from adjacent Goldner stained sections.
Results: Biopsies of 15 irradiated patients (12 men and 3 women) and 7 nonirradiated controls (5 men and 2 women) were assessed. In the study group a significant increase was seen in the number of empty lacunae, the percentage of adipose tissue of bone marrow area, and the adipocyte diameter. There was no significant difference in bone marrow fibrosis nor apoptotic osteocytes between the irradiated group and the controls.
Conclusions: Irradiation alone does not seem to induce excessive bone marrow fibrosis. The damage to bone mesenchymal stem cells leads to increased marrow adipogenesis and decreased osteoblastogenic potential. Early osteocyte death resulting in avital persisting bone matrix with severely impaired regenerative potential may contribute to the vulnerability of irradiated bone to infection and necrosis.
© 2022 The Author(s).
Figures


References
-
- Frankart AJ, Frankart MJ, Cervenka B, Tang AL, Krishnan DG, Takiar V. Osteoradionecrosis: Exposing the evidence not the bone. Int J Radiat Oncol Biol Phys. 2021;109:1206–1218. - PubMed
-
- Zhang J, Qiu X, Xi K, et al. Therapeutic ionizing radiation induced bone loss: A review of in vivo and in vitro findings. Connect Tissue Res. 2018;59:509–522. - PubMed
-
- Marx RE. Osteoradionecrosis: A new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288. - PubMed
-
- Shaw RJ, Butterworth CJ, Silcocks P, et al. HOPON (Hyperbaric Oxygen for the Prevention of Osteoradionecrosis): A randomized controlled trial of hyperbaric oxygen to prevent osteoradionecrosis of the irradiated mandible after dentoalveolar surgery. Int J Radiat Oncol Biol Phys. 2019;104:530–539. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

