Improved Cytocompatibility and Reduced Calcification of Glutaraldehyde-Crosslinked Bovine Pericardium by Modification With Glutathione
- PMID: 35662844
- PMCID: PMC9160462
- DOI: 10.3389/fbioe.2022.844010
Improved Cytocompatibility and Reduced Calcification of Glutaraldehyde-Crosslinked Bovine Pericardium by Modification With Glutathione
Abstract
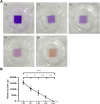
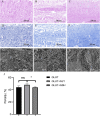
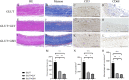
Bioprosthetic heart valves (BHVs) used in clinics are fabricated via glutaraldehyde (GLUT) crosslinking, which results in cytotoxicity and causes eventual valve calcification after implantation into the human body; therefore, the average lifetime and application of BHVs are limited. To address these issues, the most commonly used method is modification with amino acids, such as glycine (GLY), which is proven to effectively reduce toxicity and calcification. In this study, we used the l-glutathione (GSH) in a new modification treatment based on GLUT-crosslinked bovine pericardium (BP) as the GLUT + GSH group, BPs crosslinked with GLUT as GLUT-BP (control group), and GLY modification based on GLUT-BP as the GLUT + GLY group. We evaluated the characteristics of BPs in different treatment groups in terms of biomechanical properties, cell compatibility, aldehyde group content detection, and the calcification content. Aldehyde group detection tests showed that the GSH can completely neutralize the residual aldehyde group of GLUT-BP. Compared with that of GLUT-BP, the endothelial cell proliferation rate of the GLUT + GSH group increased, while its hemolysis rate and the inflammatory response after implantation into the SD rat were reduced. The results show that GSH can effectively improve the cytocompatibility of the GLUT-BP tissue. In addition, the results of the uniaxial tensile test, thermal shrinkage temperature, histological and SEM evaluation, and enzyme digestion experiments proved that GSH did not affect the ECM stability and biomechanics of the GLUT-BP. The calcification level of GLUT-BP modified using GSH technology decreased by 80%, indicating that GSH can improve the anti-calcification performance of GLUT-BP. Compared with GLUT-GLY, GLUT + GSH yielded a higher cell proliferation rate and lower inflammatory response and calcification level. GSH can be used as a new type of anti-calcification agent in GLUT crosslinking biomaterials and is expected to expand the application domain for BHVs in the future.
Keywords: anti-calcification; biomaterial modification; cytocompatibility; glutaraldehyde crosslinking; glutathione detoxification.
Copyright © 2022 Jiang, Wu, Deng, Li, Qi, Song, Liu, Wu, Xie, Chen and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bezuidenhout D., Oosthuysen A., Human P., Weissenstein C., Zilla P. (2009). The Effects of Cross-Link Density and Chemistry on the Calcification Potential of Diamine-Extended Glutaraldehyde-Fixed Bioprosthetic Heart-Valve Materials. Biotechnol. Appl. Biochem. 54, 133–140. 10.1042/BA20090101 - DOI - PubMed
LinkOut - more resources
Full Text Sources

