Vaccine effectiveness and duration of protection against symptomatic infections and severe Covid-19 outcomes in adults aged 50 years and over, France, January to mid-December 2021
- PMID: 35662871
- PMCID: PMC9135646
- DOI: 10.1016/j.gloepi.2022.100076
Vaccine effectiveness and duration of protection against symptomatic infections and severe Covid-19 outcomes in adults aged 50 years and over, France, January to mid-December 2021
Abstract
Background: SARS-CoV-2 continues to spread despite fast vaccine rollout, which could be attributed to waning immunity or to a reduced protection against some variants. A thorough characterization of vaccine protection and its duration in time is needed to inform vaccination policies and enhance public trust.
Methods: We linked three national databases with exhaustive information on screening, vaccination and hospitalizations in France from January 1st to December 12, 2021. We performed a two-step analysis to estimate vaccine effectiveness against severe outcomes of Covid-19 (requiring hospitalization) in people aged 50 years or over, combining: (i) a test-negative case-control design to assess vaccine effectiveness against symptomatic infections; and (ii) a survival analysis to assess the additional protection against severe outcomes (hospitalizations, ICU admissions and inpatient deaths) in infected individuals.
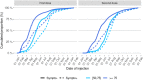
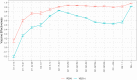
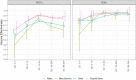
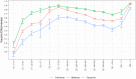
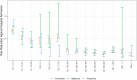
Findings: We found a high vaccine effectiveness in people aged 50 years or more, reaching 82% against symptomatic infections and 94% against hospitalizations, after a full vaccination scheme with the Covid-19 vaccines used in France.Vaccine effectiveness against symptomatic infections decreased over time, dropping to 53% after six months, but remained high against severe outcomes (90% after six months). The booster dose allowed restoring protection levels above 90% against symptomatic infections. Vaccine protection and its evolution in time, showed little difference against the variants that circulated prior to December 2021 in France, including the Delta variant.
Interpretation: Though vaccine immunity decreases over time, vaccination remains crucial to provide individual protection against severe outcomes requiring hospitalization. This decline can be reversed by the receipt of a booster dose.
Keywords: Booster; COVID-19; Effectiveness; Real-world estimates; SARS-CoV-2; Test-negative design; Vaccine.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Bouillon K., Baricault B., Botton J., Jabagi M.J., Bertrand M., Semenzato L., et al. 2021. Estimation de l’impact de la vaccination chez les personnes âgées de 75 ans et plus sur le risque de formes graves de Covid-19 en France à partir des données du Système national des données de santé (SNDS) – actualisation jusqu’au 20 juillet 2021. Rapport d’études EPI-PHARE-Groupement d’intérêt scientifique (GIS) ANSM-CNAM (octobre)
LinkOut - more resources
Full Text Sources
Miscellaneous

