The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity
- PMID: 35662937
- PMCID: PMC9157426
- DOI: 10.3389/fnut.2022.886902
The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity
Abstract
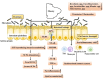
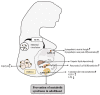
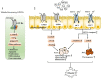
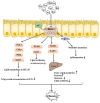
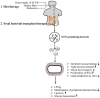
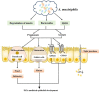
Intestinal environment disorder is a potential pathological mechanism of obesity. There is increasing evidence that disorders in the homeostasis of the intestinal environment can affect various metabolic organs, such as fat and liver, and lead to metabolic diseases. However, there are few therapeutic approaches for obesity targeting the intestinal environment. In this review, on the one hand, we discuss how intestinal microbial metabolites SCFA regulate intestinal function to improve obesity and the possible mechanisms and pathways related to obesity-related pathological processes (depending on SCFA-related receptors such as GPCRs, MCT and SMCT, and through epigenetic processes). On the other hand, we discuss dietary management strategies to enrich SCFA-producing bacteria and target specific SCFA-producing bacteria and whether fecal bacteria transplantation therapy to restore the composition of the gut microbiota to regulate SCFA can help prevent or improve obesity. Finally, we believe that it will be of great significance to establish a working model of gut- SCFA- metabolic disease development in the future for the improvement this human health concern.
Keywords: SCFA; gut microbiota; intestinal environment; metabolism; obesity.
Copyright © 2022 You, Tan, Yu, Qiu, Bai, He, Cao, Che, Guo and Su.
Conflict of interest statement
QC was employed by Guangzhou Rainhome Pharm & Tech Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

