Rhamnocitrin Attenuates Ovarian Fibrosis in Rats with Letrozole-Induced Experimental Polycystic Ovary Syndrome
- PMID: 35663203
- PMCID: PMC9162838
- DOI: 10.1155/2022/5558599
Rhamnocitrin Attenuates Ovarian Fibrosis in Rats with Letrozole-Induced Experimental Polycystic Ovary Syndrome
Abstract
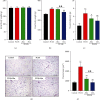
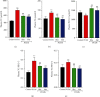
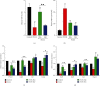
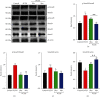
Polycystic ovary syndrome (PCOS) is a common endocrine-related cause of infertility in women and has an unknown etiology. Studies have shown that rhamnocitrin (Rha) exhibits positive effects on the reproductive system. This study investigated Rha's antifibrotic effects on PCOS rats and revealed its underlying mechanisms. Female SD rats were randomized into 4 groups (n = 8, each); the control group received tea oil by intraperitoneal injection and 1% w/v CMC by oral gavage; the PCOS group received letrozole (1 mg/kg); the PCOS+Rha group received letrozole and Rha (5 mg/kg); the PCOS+Met group received letrozole and Met (265 mg/kg) for 21 days. At the study end, Rha treatment restored letrozole-induced alterations in the relative ovarian weights, body weight, and relative weights of uterine and visceral adipose tissues. Histological observation showed that Rha ameliorates ovarian structure and fibrosis in PCOS. Administration of Rha reduced letrozole-induced metabolic dysfunction by ameliorating the levels of TC, TG, and HDL-C in the PCOS rats. Rha treatment also modulated the serum levels of sex hormones, which decreased T, E2, and LH and increased FSH in PCOS rats. In addition, Rha treatment modulated insulin resistance and increased gene expression of antioxidant enzymes (Cat, Sod2, Gpx3, Mgst1, Prdx3, Gsta4, Gsr, and Sod1) in the ovaries of the PCOS rats. Finally, Rha treatment appeared to increase the activity of PPAR-γ and inhibit the TGF-β1/Smad pathway in the ovaries of the PCOS rats. Our findings suggest that Rha significantly ameliorated metabolic disturbances and ovarian fibrosis in the PCOS rats. Rha perhaps is an effective compound for preventing ovarian fibrosis in the future.
Copyright © 2022 Yanyuan Zhou et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Dietary proanthocyanidins alleviated ovarian fibrosis in letrozole-induced polycystic ovary syndrome in rats.J Food Biochem. 2021 May;45(5):e13723. doi: 10.1111/jfbc.13723. Epub 2021 Apr 5. J Food Biochem. 2021. PMID: 33818798
-
Irpex lacteus polysaccharide exhibits therapeutic potential for ovarian fibrosis in PCOS rats via the TGF-β1/smad pathway.Heliyon. 2023 Jul 27;9(8):e18741. doi: 10.1016/j.heliyon.2023.e18741. eCollection 2023 Aug. Heliyon. 2023. PMID: 37554783 Free PMC article.
-
Sinapic acid modulates oxidative stress and metabolic disturbances to attenuate ovarian fibrosis in letrozole-induced polycystic ovary syndrome SD rats.Food Sci Nutr. 2024 Jan 24;12(4):2917-2931. doi: 10.1002/fsn3.3973. eCollection 2024 Apr. Food Sci Nutr. 2024. PMID: 38628198 Free PMC article.
-
Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: Using either hemin or L-arginine.J Cell Physiol. 2019 Jun;234(6):8426-8435. doi: 10.1002/jcp.27757. Epub 2018 Nov 15. J Cell Physiol. 2019. PMID: 30443939 Review.
-
Kuntai Capsule Combined With Letrozole on Gonadal Hormone Levels and Ovarian Function in Patients With PCOS: A Systematic Review and Meta-Analysis.Front Endocrinol (Lausanne). 2021 Dec 28;12:789909. doi: 10.3389/fendo.2021.789909. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 35027910 Free PMC article.
Cited by
-
Sinapic Acid Attenuates Chronic DSS-Induced Intestinal Fibrosis in C57BL/6J Mice by Modulating NLRP3 Inflammasome Activation and the Autophagy Pathway.ACS Omega. 2023 Dec 26;9(1):1230-1241. doi: 10.1021/acsomega.3c07474. eCollection 2024 Jan 9. ACS Omega. 2023. PMID: 38222654 Free PMC article.
-
Inhibition of FoxO1 alleviates polycystic ovarian syndrome by reducing inflammation and the immune response.Funct Integr Genomics. 2024 Jan 8;24(1):6. doi: 10.1007/s10142-024-01284-4. Funct Integr Genomics. 2024. PMID: 38189995
-
The mechanism of Leonuri Herba in improving polycystic ovary syndrome was analyzed based on network pharmacology and molecular docking.J Pharm Pharm Sci. 2023 Feb 15;26:11234. doi: 10.3389/jpps.2023.11234. eCollection 2023. J Pharm Pharm Sci. 2023. PMID: 36942296 Free PMC article.
-
Natural compounds in the management of polycystic ovary syndrome: a comprehensive review of hormonal regulation and therapeutic potential.Front Nutr. 2025 Feb 11;12:1520695. doi: 10.3389/fnut.2025.1520695. eCollection 2025. Front Nutr. 2025. PMID: 40008316 Free PMC article. Review.
-
Extracellular matrix dysregulation in PCOS: pathogenesis, therapeutic strategies, and innovative technologies.J Biol Eng. 2025 Jul 5;19(1):61. doi: 10.1186/s13036-025-00533-9. J Biol Eng. 2025. PMID: 40618169 Free PMC article. Review.
References
-
- González F., Considine R. V., Abdelhadi O. A., Acton A. J. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism . 2019;104(11):5360–5371. doi: 10.1210/jc.2019-00987. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

