Icaritin inhibits PLK1 to activate DNA damage response in NK/T cell lymphoma and increases sensitivity to GELOX regime
- PMID: 35663228
- PMCID: PMC9127125
- DOI: 10.1016/j.omto.2022.04.012
Icaritin inhibits PLK1 to activate DNA damage response in NK/T cell lymphoma and increases sensitivity to GELOX regime
Abstract
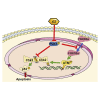
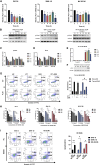
Natural killer/T cell lymphoma (NKTCL) is a highly aggressive subtype of non-Hodgkin lymphoma. Gemcitabine, oxaliplatin, and L-asparaginase (GELOX) is one of the first-line chemotherapy regimens of NKTCL. Yet, the prognosis of NKTCL is poor. Icaritin is an herb-derived monomer from icariin with antitumor effects. We found that icaritin induced proliferation inhibition and apoptosis of NKTCL both in vitro and in vivo. Moreover, icaritin inhibited the dissemination of NKTCL in vivo. RNA sequencing revealed the Polo-like kinase 1 (PLK1) gene and DNA damage response (DDR) as the targets of icaritin. Mechanistically, icaritin inhibited PLK1 to promote checkpoint kinase 2 (Chk2) homodimerization and its T387 phosphorylation, which further activated p53, leading to the activation of the DDR pathway. Moreover, inhibiting PLK1 increased Forkhead box O3a nuclear localization, the latter of which activated ataxia telangiectasia mutated (ATM), an early sensor of DNA damage. Then ATM phosphorylated Chk2 T68 and initiated Chk2 activation. Remarkably, the combined treatment of icaritin and GELOX achieved better antitumor efficacy than single treatment in vivo. In summary, our results proved the efficacy of icaritin treating NKTCL, provided insights into its antitumor molecular mechanism, and revealed the application value of icaritin in facilitating clinical NKTCL treatment.
Keywords: Chk2; DNA damage response; FOXO3a; NKTCL; PLK1; icaritin.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Swerdlow S.H.C.E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J. Fourth edition. IARC; 2017. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised.
-
- Yamaguchi M., Kwong Y.L., Kim W.S., Maeda Y., Hashimoto C., Suh C., Izutsu K., Ishida F., Isobe Y., Sueoka E., et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group Study. J. Clin. Oncol. 2011;29:4410–4416. doi: 10.1200/jco.2011.35.6287. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

