Catalytic Reductive Carbene Transfer Reactions
- PMID: 35663346
- PMCID: PMC9159636
- DOI: 10.1016/j.checat.2022.01.002
Catalytic Reductive Carbene Transfer Reactions
Abstract
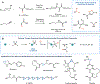
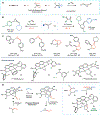
Efforts to develop catalytic carbene transfer reactions have largely relied on the use of diazo precursors. However, diazoalkanes are susceptible to undergoing violent exothermic decomposition unless they contain stabilizing substituents. Consequently, most synthetic methods are restricted to diazoacetates or related derivatives. In this Perspective, we describe an alternative approach to carbene transfer catalysis based on the generation of metal carbenoids from gem-dihaloalkanes and gem-dihaloalkenes. These precursors are readily available and stable in unsubstituted form or with a variety of donor and acceptor substituents. Using this approach, it is possible to design cyclopropanation reactions with non-stabilized carbenes, such as methylene, isopropylidene, and vinylidene. Furthermore, due to the distinct mechanistic pathways of these reactions, novel modes of cycloaddition can be carried out, including [4 + 1]-cycloadditions.
Keywords: carbenes; cycloadditions; transition metal catalysis.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures



References
-
- Silberrad O, and Roy CS (1906). XXIV.—Gradual decomposition of ethyl diazoacetate. J. Chem Soc., Trans 89, 179–182. 10.1039/CT9068900179. - DOI
-
- Nozaki H, Moriuti S, Yamabe M, and Noyori R (1966). Reactions of diphenyldiazomethane in the presence of bis (acetylacetonato) copper (II). Modified diphenylmethylene reactions. Tetrahedron Lett. 7, 59–63.
-
- Moser WR (1969). Mechanism of the copper-catalyzed addition of diazoalkanes to olefins. I. Steric effects. J. Am. Chem. Soc. 91, 1135–1140. 10.1021/ja01033a017. - DOI
-
- Nozaki H, Moriuti S, Takaya H, and Noyori R (1966). Asymmetric induction in carbenoid reaction by means of a dissymmetric copper chelate. Tetrahedron Lett. 7, 5239–5244. 10.1016/S0040-4039(01)89263-7. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
